OlvanilPotent vanilloid receptor agonist CAS# 58493-49-5 |
- Cefditoren Pivoxil
Catalog No.:BCC4898
CAS No.:117467-28-4
- Balofloxacin
Catalog No.:BCC4892
CAS No.:127294-70-6
- Pefloxacin Mesylate Dihydrate
Catalog No.:BCC5089
CAS No.:149676-40-4
- Tinidazole
Catalog No.:BCC4866
CAS No.:19387-91-8
- Toltrazuril
Catalog No.:BCC4870
CAS No.:69004-03-1
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 58493-49-5 | SDF | Download SDF |
| PubChem ID | 5808649 | Appearance | Powder |
| Formula | C26H43NO3 | M.Wt | 417.63 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | NE 19550 | ||
| Solubility | Soluble to 100 mM in DMSO and to 25 mM in ethanol | ||
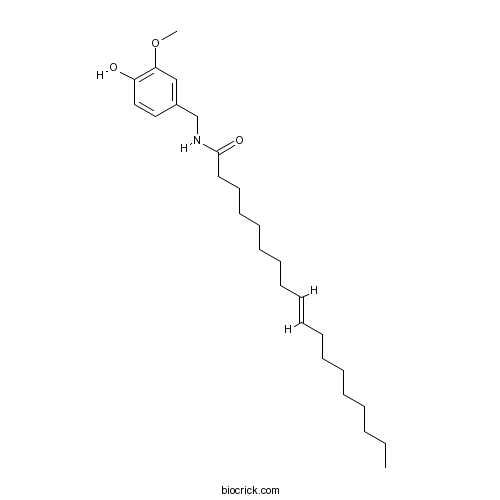
| Chemical Name | (E)-N-[(4-hydroxy-3-methoxyphenyl)methyl]octadec-9-enamide | ||
| SMILES | CCCCCCCCC=CCCCCCCCC(=O)NCC1=CC(=C(C=C1)O)OC | ||
| Standard InChIKey | OPZKBPQVWDSATI-ZHACJKMWSA-N | ||
| Standard InChI | InChI=1S/C26H43NO3/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-26(29)27-22-23-19-20-24(28)25(21-23)30-2/h10-11,19-21,28H,3-9,12-18,22H2,1-2H3,(H,27,29)/b11-10+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent vanilloid receptor agonist (pEC50 values are 8.1 and 7.7 at rat and human VR1 receptors respectively). Also blocks anandamide uptake (IC50 = 9 μM) and may bind to CB1 cannabinoid receptors. Antinociceptive following systemic administration. Also available as part of the Vanilloid TRPV1 Receptor. |

Olvanil Dilution Calculator

Olvanil Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3945 mL | 11.9723 mL | 23.9446 mL | 47.8893 mL | 59.8616 mL |
| 5 mM | 0.4789 mL | 2.3945 mL | 4.7889 mL | 9.5779 mL | 11.9723 mL |
| 10 mM | 0.2394 mL | 1.1972 mL | 2.3945 mL | 4.7889 mL | 5.9862 mL |
| 50 mM | 0.0479 mL | 0.2394 mL | 0.4789 mL | 0.9578 mL | 1.1972 mL |
| 100 mM | 0.0239 mL | 0.1197 mL | 0.2394 mL | 0.4789 mL | 0.5986 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Lemannine
Catalog No.:BCN3742
CAS No.:58480-54-9
- Platycodin D
Catalog No.:BCN4982
CAS No.:58479-68-8
- Ferrugine
Catalog No.:BCN1910
CAS No.:58471-11-7
- Darlingine
Catalog No.:BCN1906
CAS No.:58471-10-6
- N-(2-Hydroxy-4-methoxyphenyl)acetamide
Catalog No.:BCN1409
CAS No.:58469-06-0
- H-D-Leu-OMe.HCl
Catalog No.:BCC2681
CAS No.:5845-53-4
- Angiotensin 1/2 (1-5)
Catalog No.:BCC1035
CAS No.:58442-64-1
- Boc-2-Nal-OH
Catalog No.:BCC3289
CAS No.:58438-04-3
- H-2-Nal-OH.HCl
Catalog No.:BCC3287
CAS No.:58438-03-2
- Dihydroresveratrol
Catalog No.:BCN5793
CAS No.:58436-28-5
- DL-Demethylcoclaurine
Catalog No.:BCC8317
CAS No.:5843-65-2
- Isopimaric acid
Catalog No.:BCN4618
CAS No.:5835-26-7
- ent-16beta,17-Isopropylidenedioxykaurane
Catalog No.:BCN1408
CAS No.:58493-71-3
- IOX 1
Catalog No.:BCC6192
CAS No.:5852-78-8
- Pseurotin A
Catalog No.:BCN7246
CAS No.:58523-30-1
- (-)-Cephaeline dihydrochloride
Catalog No.:BCN8323
CAS No.:5853-29-2
- Rebaudioside A
Catalog No.:BCN5900
CAS No.:58543-16-1
- Rebaudioside B
Catalog No.:BCN2612
CAS No.:58543-17-2
- Cucurbitacin IIA
Catalog No.:BCN5019
CAS No.:58546-34-2
- Gomisin A
Catalog No.:BCN5794
CAS No.:58546-54-6
- Schisantherin B
Catalog No.:BCN1023
CAS No.:58546-55-7
- Schisantherin A
Catalog No.:BCN1024
CAS No.:58546-56-8
- Confluentin
Catalog No.:BCN5795
CAS No.:585534-03-8
- Losmapimod
Catalog No.:BCC5368
CAS No.:585543-15-3
Non-pungent long chain capsaicin-analogs arvanil and olvanil display better anti-invasive activity than capsaicin in human small cell lung cancers.[Pubmed:27196129]
Cell Adh Migr. 2017 Jan 2;11(1):80-97.
The nutritional compound capsaicin inhibits the invasion of many types of human cancers. The clinical development of capsaicin as an anti-cancer drug is limited due to its unfavorable side effects like burning sensation, stomach cramps, gut pain and nausea. This study compared the anti-invasive activity of capsaicin to non-pungent long chain capsaicin analogs, namely arvanil and Olvanil, in human small cell lung cancer cells. Boyden chamber invasion assays revealed that arvanil and Olvanil displayed improved anti-invasive activity relative to capsaicin in human SCLC cells. The results of the Boyden chamber assay were confirmed by the spherical invasion assay, and similar results were obtained. The anti-invasive activity of arvanil, Olvanil and capsaicin were independent of TRPV and CB1 receptors. Furthermore, the anti-invasive activity of arvanil, Olvanil and capsaicin was mediated by the AMPK pathway. Depletion of AMPK levels by siRNA methodology abrogated the anti-invasive activity of arvanil, Olvanil and capsaicin. The non-pungent capsaicin analogs arvanil and Olvanil display improved anti-invasive activity relative to capsaicin in human SCLC cells. These agents may represent the second generation of capsaicin-like compounds which are more potent than the parent molecule and have a better side effect profile.
Anti-nociceptive and desensitizing effects of olvanil on capsaicin-induced thermal hyperalgesia in the rat.[Pubmed:27439609]
BMC Pharmacol Toxicol. 2016 Jul 21;17(1):31.
BACKGROUND: Olvanil (NE 19550) is a non-pungent synthetic analogue of capsaicin, the natural pungent ingredient of capsicum which activates the transient receptor potential vanilloid type-1 (TRPV1) channel and was developed as a potential analgesic compound. Olvanil has potent anti-hyperalgesic effects in several experimental models of chronic pain. Here we report the inhibitory effects of Olvanil on nociceptive processing using cultured dorsal root ganglion (DRG) neurons and compare the effects of capsaicin and Olvanil on thermal nociceptive processing in vivo; potential contributions of the cannabinoid CB1 receptor to Olvanil's anti-hyperalgesic effects were also investigated. METHODS: A hot plate analgesia meter was used to evaluate the anti-nociceptive effects of Olvanil on capsaicin-induced thermal hyperalgesia and the role played by CB1 receptors in mediating these effects. Single cell calcium imaging studies of DRG neurons were employed to determine the desensitizing effects of Olvanil on capsaicin-evoked calcium responses. Statistical analysis used Student's t test or one way ANOVA followed by Dunnett's post-hoc test as appropriate. RESULTS: Both Olvanil (100 nM) and capsaicin (100 nM) produced significant increases in intracellular calcium concentrations [Ca(2+)]i in cultured DRG neurons. Olvanil was able to desensitise TRPV1 responses to further capsaicin exposure more effectively than capsaicin. Intraplantar injection of capsaicin (0.1, 0.3 and 1 mug) produced a robust TRPV1-dependant thermal hyperalgesia in rats, whilst Olvanil (0.1, 0.3 and 1 mug) produced no hyperalgesia, emphasizing its lack of pungency. The highest dose of Olvanil significantly reduced the hyperalgesic effects of capsaicin in vivo. Intraplantar injection of the selective cannabinoid CB1 receptor antagonist rimonabant (1 mug) altered neither capsaicin-induced thermal hyperalgesia nor the desensitizing properties of Olvanil, indicating a lack of involvement of CB1 receptors. CONCLUSIONS: Olvanil is effective in reducing capsaicin-induced thermal hyperalgesia, probably via directly desensitizing TRPV1 channels in a CB1 receptor-independent fashion. The results presented clearly support the potential for Olvanil in the development of new topical analgesic preparations for treating chronic pain conditions while avoiding the unwanted side effects of capsaicin treatments.
Olvanil acts on transient receptor potential vanilloid channel 1 and cannabinoid receptors to modulate neuronal transmission in the trigeminovascular system.[Pubmed:22902197]
Pain. 2012 Nov;153(11):2226-32.
The transient receptor potential vanilloid channel 1 (TRPV1) is a nociceptive transducer located on nociceptive neurons. TRPV1 channels located on peripheral neurons mainly transduce the sense of heat and are also activated by low pH or capsaicin. The role of centrally located TRPV1 channels is not fully understood. Likewise their importance in pain syndromes of central origin, such as migraine, is not known. Experimental data suggest a relationship to migraine. However, experimental studies with TRPV1 receptor antagonists indicate that the receptor may not be a useful target for new acute migraine treatments. Any potential role for the receptor in the chronification of migraine has not been investigated. The present study aimed at analyzing the use of the TRPV1 channel as a target to desensitize trigeminal neurons and thereby inhibit neuronal activity in the trigeminocervical complex. The TRPV1 receptor agonist Olvanil was used for desensitization because, as compared with capsaicin, it is non-noxious and lacks capsaicin's pungency and CGRP release potential. We further investigated a possible effect of Olvanil on cannabinoid (CB(1)) receptors, as an interaction between both receptor systems has been described previously. The results show that Olvanil dose-dependently inhibited spontaneous and stimulus-induced activity within the trigeminocervical complex, whereas it had no effect on CSD susceptibility. We further demonstrated that the inhibiting effect of Olvanil is mediated by vanilloid and cannabinoid receptor systems, thereby using the synergistic effects this dual mechanism offers. Curiously, TRPV1 receptor agonism may have anti-nociceptive properties through central mechanisms that would be of considerable interest to elucidate.
TRPV1 agonist piperine but not olvanil enhances glutamatergic spontaneous excitatory transmission in rat spinal substantia gelatinosa neurons.[Pubmed:21703243]
Biochem Biophys Res Commun. 2011 Jul 15;410(4):841-5.
We examined the effects of TRPV1 agonists Olvanil and piperine on glutamatergic spontaneous excitatory transmission in the substantia gelatinosa (SG) neurons of adult rat spinal cord slices with the whole-cell patch-clamp technique. Bath-applied Olvanil did not affect the frequency and amplitude of spontaneous excitatory postsynaptic current (sEPSC), and unchanged holding currents at -70 mV. On the other hand, superfusing piperine reversibly and concentration-dependently increased sEPSC frequency (half-maximal effective concentration: 52.3 muM) with a minimal increase in its amplitude. This sEPSC frequency increase was almost repetitive at an interval of more than 20 min. Piperine at a high concentration produced an inward current in some neurons. The facilitatory effect of piperine was blocked by TRPV1 antagonist capsazepine. It is concluded that piperine but not Olvanil activates TRPV1 channels in the central terminals of primary-afferent neurons, resulting in an increase in the spontaneous release of l-glutamate onto SG neurons.
Cannabinoid activation of recombinant and endogenous vanilloid receptors.[Pubmed:11672565]
Eur J Pharmacol. 2001 Jul 27;424(3):211-9.
The effects of three structurally related cannabinoids on human and rat recombinant vanilloid VR1 receptors expressed in human embryonic kidney (HEK293) cells and at endogenous vanilloid receptors in the rat isolated mesenteric arterial bed were studied. In the recombinant cells, all three were full agonists, causing concentration-dependent increases in [Ca(2+)](i) (FLIPR), with a rank order of potency relative to the vanilloids capsaicin and Olvanil, of Olvanil> or =capsaicin>AM404 ((allZ)-N-(4-hydroxyphenyl)-5,8,11,14-eicosatetraenamide)>anandamide>methanandami de. These responses were inhibited by the vanilloid VR1 receptor antagonist, capsazepine. In the mesenteric arterial bed, vasorelaxation was evoked by these ligands with a similar order of potency. The AM404-induced vasorelaxation was virtually abolished by capsaicin pretreatment. AM404 inhibition of capsaicin-sensitive sensory neurotransmission was blocked by ruthenium red, but not by cannabinoid CB(1) and CB(2) receptor antagonists. AM404 had no effect on relaxations to calcitonin gene-related peptide. These data demonstrate that the vasorelaxant and sensory neuromodulator properties of AM404 in the rat isolated mesenteric arterial bed are mediated by vanilloid VR1 receptors.
Interactions between synthetic vanilloids and the endogenous cannabinoid system.[Pubmed:9801167]
FEBS Lett. 1998 Oct 9;436(3):449-54.
The chemical similarity between some synthetic agonists of vanilloid receptors, such as Olvanil (N-vanillyl-cis-9-octadecenoamide), and the 'endocannabinoid' anandamide (arachidonoyl-ethanolamide, AEA), suggests possible interactions between the cannabinoid and vanilloid signalling systems. Here we report that Olvanil is a stable and potent inhibitor of AEA facilitated transport into rat basophilic leukemia (RBL-2H3) cells. Olvanil blocked both the uptake and the hydrolysis of [14C]AEA by intact RBL-2H3 cells (IC50 = 9 microM), while capsaicin and pseudocapsaicin (N-vanillyl-nonanamide) were much less active. Olvanil was more potent than previously reported inhibitors of AEA facilitated transport, i.e. phloretin (IC50 = 80 microM), AM404 (12.9% inhibition at 10 microM) or oleoylethanolamide (27.5% inhibition at 10 microM). Olvanil was a poor inhibitor of [14C]AEA hydrolysis by RBL-2H3 and N18TG2 cell membranes, suggesting that the inhibitory effect on [14C]AEA breakdown observed in intact cells was due to inhibition of [14C]AEA uptake. Olvanil was stable to enzymatic hydrolysis, and (i) displaced the binding of high affinity cannabinoid receptor ligands to membrane preparations from N18TG2 cells and guinea pig forebrain (Ki = 1.64-7.08 microM), but not from cells expressing the CB2 cannabinoid receptor subtype; (ii) inhibited forskolin-induced cAMP formation in intact N18TG2 cells (IC50 = 1.60 microM), this effect being reversed by the selective CB1 antagonist SR141716A. Pseudocapsaicin, but not capsaicin, also selectively bound to CB1 receptor-containing membranes. These data suggest that some of the analgesic actions of Olvanil may be due to its interactions with the endogenous cannabinoid system, and may lead to the design of a novel class of cannabimimetics with potential therapeutic applications as analgesics.
Vanilloids. 1. Analogs of capsaicin with antinociceptive and antiinflammatory activity.[Pubmed:8410971]
J Med Chem. 1993 Sep 3;36(18):2595-604.
As part of a program to establish structure-activity relationships for vanilloids, analogs of the pungent principle capsaicin, the alkyl chain portion of the parent structure (and related compounds derived from homovanillic acid) was varied. In antinociceptive and antiinflammatory assays (rat and mouse hot plate and croton oil-inflamed mouse ear), compounds with widely varying alkyl chain structures were active. Short-chain compounds were active by systemic administration in the assays mentioned above but they retained the high pungency and acute toxicity characteristic of capsaicin. In contrast, the long chain cis-unsaturates, NE-19550 (vanillyloleamide) and NE-28345 (oleylhomovanillamide), were orally active, less pungent, and less acutely toxic than capsaicin. The potential of these compounds as antiinflammatory/analgesic agents is discussed in light of recent data on the mechanism of action of vanilloids on sensory nerve fibers.
NE-19550 and NE-21610, antinociceptive capsaicin analogues: studies on nociceptive fibres of the neonatal rat tail in vitro.[Pubmed:2384136]
Eur J Pharmacol. 1990 Jun 8;181(3):289-93.
When applied to peripheral fibres in a neonatal rat tail/spinal cord preparation in vitro, capsaicin (0.2-50 microM) induced an activation, selective desensitization and reduced responses to other noxious stimuli (heat, bradykinin). Similar concentrations of the antinociceptive analogues NE-19550 and NE-21610, did not affect peripheral fibre responsiveness but induced cross desensitization to capsaicin. At 500 microM both analogues produced similar effects to capsaicin. Capsaicin analogues may induce analgesia without initial activation of nociceptors.


