NiazirinCAS# 122001-32-5 |
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 122001-32-5 | SDF | Download SDF |
| PubChem ID | 129556 | Appearance | Powder |
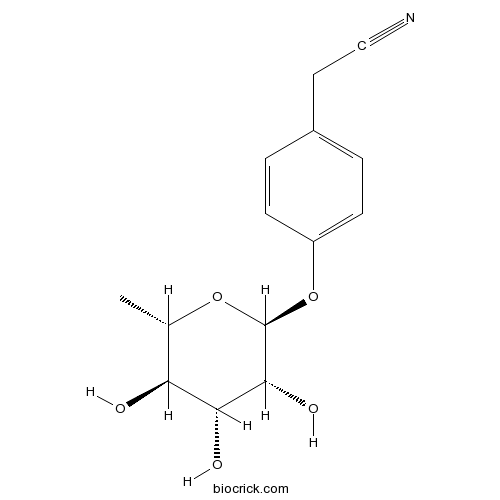
| Formula | C14H17NO5 | M.Wt | 279.29 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 2-[4-[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxyphenyl]acetonitrile | ||
| SMILES | CC1C(C(C(C(O1)OC2=CC=C(C=C2)CC#N)O)O)O | ||
| Standard InChIKey | OBJREHLZEIEGDU-CNJBRALLSA-N | ||
| Standard InChI | InChI=1S/C14H17NO5/c1-8-11(16)12(17)13(18)14(19-8)20-10-4-2-9(3-5-10)6-7-15/h2-5,8,11-14,16-18H,6H2,1H3/t8-,11-,12+,13+,14-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. The Moringa oleifera extract(including niaziridin and Niazirin) can successfully attenuate the development of pulmonary hypertension via direct vasodilatation and a potential increase in antioxidant activity. |

Niazirin Dilution Calculator

Niazirin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.5805 mL | 17.9025 mL | 35.8051 mL | 71.6102 mL | 89.5127 mL |
| 5 mM | 0.7161 mL | 3.5805 mL | 7.161 mL | 14.322 mL | 17.9025 mL |
| 10 mM | 0.3581 mL | 1.7903 mL | 3.5805 mL | 7.161 mL | 8.9513 mL |
| 50 mM | 0.0716 mL | 0.3581 mL | 0.7161 mL | 1.4322 mL | 1.7903 mL |
| 100 mM | 0.0358 mL | 0.179 mL | 0.3581 mL | 0.7161 mL | 0.8951 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Sulfamonomethoxine
Catalog No.:BCC9156
CAS No.:1220-83-3
- 3-Phenyl-1-propanol
Catalog No.:BCC8102
CAS No.:122-97-4
- Phenylacetaldehyde
Catalog No.:BCN3819
CAS No.:122-78-1
- Cinnamyl cinnamate
Catalog No.:BCN4722
CAS No.:122-69-0
- (-)-Ampelopsin H
Catalog No.:BCC8842
CAS No.:
- Zingerone
Catalog No.:BCN1192
CAS No.:122-48-5
- Glycerine trioleate
Catalog No.:BCN2287
CAS No.:122-32-7
- Tetraethoxypropane
Catalog No.:BCN2221
CAS No.:122-31-6
- Sulfadimethoxine
Catalog No.:BCC5159
CAS No.:122-11-2
- (±)-Anatoxin A fumarate
Catalog No.:BCC6796
CAS No.:1219922-30-1
- PF 4778574
Catalog No.:BCC6322
CAS No.:1219633-99-4
- 4,5-Diepipsidial A
Catalog No.:BCN3920
CAS No.:1219603-97-0
- Cyhalofop
Catalog No.:BCC5474
CAS No.:122008-78-0
- PLP (139-151)
Catalog No.:BCC5920
CAS No.:122018-58-0
- Monomethyl lithospermate B
Catalog No.:BCN2533
CAS No.:122021-74-3
- Khayalenoid E
Catalog No.:BCN6111
CAS No.:1220508-29-1
- [bAla8]-Neurokinin A(4-10)
Catalog No.:BCC7137
CAS No.:122063-01-8
- 2'-O-Acetylsprengerinin C
Catalog No.:BCN6655
CAS No.:1220707-33-4
- Charantadiol A
Catalog No.:BCN3483
CAS No.:1220890-23-2
- 3,4-Dihydro-3,4-dihydroxynaphthalen-1(2H)-one
Catalog No.:BCN1602
CAS No.:1220891-22-4
- Auraptenol
Catalog No.:BCN6113
CAS No.:1221-43-8
- 2-Deoxy-2,2-difluoro-D-erythro-pentafuranous-1-ulose-3,5-dibenzoate
Catalog No.:BCC8575
CAS No.:122111-01-7
- Gemcitabine HCl
Catalog No.:BCC1076
CAS No.:122111-03-9
- ER-878898
Catalog No.:BCC8958
CAS No.:122111-11-9
An antitumor promoter from Moringa oleifera Lam.[Pubmed:10209341]
Mutat Res. 1999 Apr 6;440(2):181-8.
In the course of studies on the isolation of bioactive compounds from Philippine plants, the seeds of Moringa oleifera Lam. were examined and from the ethanol extract were isolated the new O-ethyl-4-(alpha-L-rhamnosyloxy)benzyl carbamate (1) together with seven known compounds, 4(alpha-L-rhamnosyloxy)-benzyl isothiocyanate (2), niazimicin (3), Niazirin (4), beta-sitosterol (5), glycerol-1-(9-octadecanoate) (6), 3-O-(6'-O-oleoyl-beta-D-glucopyranosyl)-beta-sitosterol (7), and beta-sitosterol-3-O-beta-D-glucopyranoside (8). Four of the isolates (2, 3, 7, and 8), which were obtained in relatively good yields, were tested for their potential antitumor promoting activity using an in vitro assay which tested their inhibitory effects on Epstein-Barr virus-early antigen (EBV-EA) activation in Raji cells induced by the tumor promoter, 12-O-tetradecanoyl-phorbol-13-acetate (TPA). All the tested compounds showed inhibitory activity against EBV-EA activation, with compounds 2, 3 and 8 having shown very significant activities. Based on the in vitro results, niazimicin (3) was further subjected to in vivo test and found to have potent antitumor promoting activity in the two-stage carcinogenesis in mouse skin using 7,12-dimethylbenz(a)anthracene (DMBA) as initiator and TPA as tumor promoter. From these results, niazimicin (3) is proposed to be a potent chemo-preventive agent in chemical carcinogenesis.
Isolation and structure elucidation of new nitrile and mustard oil glycosides from Moringa oleifera and their effect on blood pressure.[Pubmed:7798960]
J Nat Prod. 1994 Sep;57(9):1256-61.
Bioassay-guided analysis of an EtOH extract of Moringa oleifera leaves showing hypotensive activity led to the isolation of two nitrile glycosides, Niazirin [1] and Niazirinin [2], and three mustard oil glycosides, 4-[(4'-O-acetyl-alpha-L-rhamnosyloxy)benzyl]isothiocyanate [4], niaziminin A, and niaziminin B. Glycoside 2 is a new compound. Niaziminins A and B have previously been obtained from the left extract as a mixture, while compound 4 is new from this source. Structural determination was accomplished by means of spectroscopic methods including appropriate 2D nmr experiments and chemical reactions. This is the first report of the isolation of nitriles, an isothiocyanate, and thiocarbamates from the same plant species. Isothiocyanate 4 and the thiocarbamate glycosides niaziminin A and B showed hypotensive activity while nitrile glycosides 1 and 2 were found to be inactive in this regard.
Effects of 2-azafluorenones on phosphatidyl-inositol specific phospholipase C activation in c6 glioma cells.[Pubmed:22559734]
Chin J Physiol. 2012 Apr 30;55(2):101-7.
The purpose of this study was to determine the effects of an extract from Moringa oleifera (MO) on the development of monocrotaline (MCT)-induced pulmonary hypertension (PH) in Wistar rats. An ethanol extraction was performed on dried MO leaves, and HPLC analysis identified niaziridin and Niazirin in the extract. PH was induced with a single subcutaneous injection of MCT (60 mg/kg) which resulted in increases in pulmonary arterial blood pressure (Ppa) and in thickening of the pulmonary arterial medial layer in the rats. Three weeks after induction, acute administration of the MO extract to the rats decreased Ppa in a dose-dependent manner that reached statistical significance at a dose of 4.5 mg of freeze-dried extract per kg body weight. The reduction in Ppa suggested that the extract directly relaxed the pulmonary arteries. To assay the effects of chronic administration of the MO extract on PH, control, MCT and MCT+MO groups were designated. Rats in the control group received a saline injection; the MCT and MCT+MO groups received MCT to induce PH. During the third week after MCT treatment, the MCT+MO group received daily i.p. injections of the MO extract (4.5 mg of freeze-dried extract/kg of body weight). Compared to the control group, the MCT group had higher Ppa and thicker medial layers in the pulmonary arteries. Chronic treatments with the MO extract reversed the MCT-induced changes. Additionally, the MCT group had a significant elevation in superoxide dismutase activity when normalized by the MO extract treatments. In conclusion, the MO extract successfully attenuated the development of PH via direct vasodilatation and a potential increase in antioxidant activity.
Unusual glycosides of pyrrole alkaloid and 4'-hydroxyphenylethanamide from leaves of Moringa oleifera.[Pubmed:21439596]
Phytochemistry. 2011 Jun;72(8):791-5.
Glycosides of pyrrole alkaloid (pyrrolemarumine 4''-O-alpha-L-rhamnopyranoside) and 4'-hydroxyphenylethanamide (marumosides A and B) were isolated from leaves of Moringa oleifera along with eight known compounds; Niazirin, methyl 4-(alpha-L-rhamnopyranosyloxy)benzylcarbamate, benzyl beta-D-glucopyranoside, benzyl beta-D-xylopyranosyl-(1-->6)-beta-D-glucopyranoside, kaempferol 3-O-beta-D-glucopyranoside, quercetin 3-O-beta-D-glucopyranoside, adenosine and L-tryptophan. Structure elucidations were based on analyses of chemical and spectroscopic data including 1D- and 2D-NMR.
Review: an exposition of medicinal preponderance of Moringa oleifera (Lank.).[Pubmed:24577932]
Pak J Pharm Sci. 2014 Mar;27(2):397-403.
Medicinal plants are believed to be a precious natural reservoir as they are assumed to have paranormal effects for the mankind. Moringa oleifera grows throughout most of the tropics and has numerous industrial and medicinal uses. This review acquaints with the consequence of fera (Moringaceae), a fast growing medicinal plant wide spread in tropical regions with height ranging from 5-10m. It has an enormous nutritional worth due to existence of vitamins and proteins. It is subsisted with many constituents. Its oil consists of oleic, tocopherols, stearic, palmitic, behenic and arachidic acid. Flavanoids and phenolics such as gallic acid, chlorogenic acid, ferulic acid, kaempferol, ellagic acid, quercetin and vanillin are present by means of leaf extract, being richest in phenolics and subsequent fruit and seed extract respectively, that are accountable for antioxidant activity of plant. Seeds have been pragmatic with active components as novel O-ethyl-4- (alpha -L-rhamnosyloxy) benzyl carbamate together with seven known compounds, 4 (alpha -L-rhamnosyloxy)-benzyl isothiocyanate, niazimicin, Niazirin, beta-sitosterol, glycerol-1- (9 -octadecanoate), 3 -O- 6 -O-oleoyl- beta -D-glucopyranosyl-b-sitosterol, and beta - sitosterol- 3-X-O -beta -D-glucopyranoside , that have been discerned to inhibit EBV-EA (Epstein- Barr virus-early antigen), that is persuaded by the cancer promoter. M. oleifera leaves, gums, roots, flowers as well as kernels have been unanimously utilized for managing tissue tenderness, cardiovascular and liver maladies, normalize blood glucose and cholesterol. It has also profound antimicrobial, hypoglycemic and anti-tubercular activities.
Attenuation of the extract from Moringa oleifera on monocrotaline-induced pulmonary hypertension in rats.[Pubmed:22242951]
Chin J Physiol. 2012 Feb 29;55(1):22-30.
The purpose of this study was to determine the effects of an extract from Moringa oleifera (MO) on the development of monocrotaline (MCT)-induced pulmonary hypertension (PH) in Wistar rats. An ethanol extraction was performed on dried MO leaves, and HPLC analysis identified niaziridin and Niazirin in the extract. PH was induced with a single subcutaneous injection of MCT (60 mg/kg) which resulted in increases in pulmonary arterial blood pressure (Ppa) and in thickening of the pulmonary arterial medial layer in the rats. Three weeks after induction, acute administration of the MO extract to the rats decreased Ppa in a dose-dependent manner that reached statistical significance at a dose of 4.5 mg of freeze-dried extract per kg body weight. The reduction in Ppa suggested that the extract directly relaxed the pulmonary arteries. To assay the effects of chronic administration of the MO extract on PH, control, MCT and MCT+MO groups were designated. Rats in the control group received a saline injection; the MCT and MCT+MO groups received MCT to induce PH. During the third week after MCT treatment, the MCT+MO group received daily i.p. injections of the MO extract (4.5 mg of freeze-dried extract/kg of body weight). Compared to the control group, the MCT group had higher Ppa and thicker medial layers in the pulmonary arteries. Chronic treatments with the MO extract reversed the MCT-induced changes. Additionally, the MCT group had a significant elevation in superoxide dismutase activity when normalized by the MO extract treatments. In conclusion, the MO extract successfully attenuated the development of PH via direct vasodilatation and a potential increase in antioxidant activity.
[Constituents isolated from n-butanol extract of leaves of Moringa oleifera].[Pubmed:29552820]
Zhongguo Zhong Yao Za Zhi. 2018 Jan;43(1):114-118.
Seventeen compounds were isolated from n-butanol extract of the leaves of Moringa oleifera, using column chromatography over macroporous resin HP-20,Sephadex LH-20, and ODS. Their structures were identified as two carboline,tangutorid E(1) and tangutorid F(2); three phenolic glycosides,Niazirin(3)benzaldehyde 4-O-alpha-L-rhamnopyranoside(4) and 4-O-beta-D-glucopyranosidebenzoic acid(5); four chlorogenic acid and derivatives,4-caffeoylquinic acid(6),methyl 4-caffeoylquinate(7),caffeoylquinic acid(8) and methyl caffeoylquinate(9); two nucleosids,uridine(10) and adenosine(11); one flavone,quercetin 3-O-beta-D-glucopyranoside(12); five other types of compounds,phthalimidineacetic acid(13),3-pyridinecarboxamide(14),3,4-dihydroxy-benzoic acid(15),5-hydroxymethyl-2-furancarboxylic acid(16) and 5-hydroxymethyl-2-furaldehyde(17) by the spectral data of (1)H, (1)(3)C-NMR and MS. Among them,compounds 1-2,7,9-10,16 and 17 were isolated from M. oleifera for the first time.


