NF 023Selective, competitive P2X1 antagonist CAS# 104869-31-0 |
- Tubastatin A HCl
Catalog No.:BCC3877
CAS No.:1310693-92-5
- Entinostat (MS-275,SNDX-275)
Catalog No.:BCC3595
CAS No.:209783-80-2
- M344
Catalog No.:BCC2162
CAS No.:251456-60-7
- Panobinostat (LBH589)
Catalog No.:BCC3601
CAS No.:404950-80-7
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 104869-31-0 | SDF | Download SDF |
| PubChem ID | 4465 | Appearance | Powder |
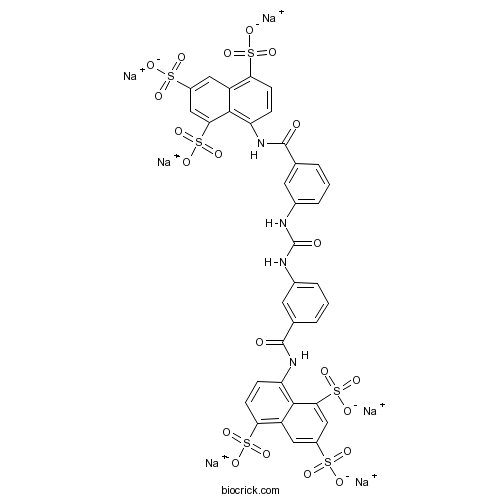
| Formula | C35H20N4Na6O21S6 | M.Wt | 1162.86 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in water | ||
| Chemical Name | hexasodium;8-[[3-[[3-[(4,6,8-trisulfonatonaphthalen-1-yl)carbamoyl]phenyl]carbamoylamino]benzoyl]amino]naphthalene-1,3,5-trisulfonate | ||
| SMILES | C1=CC(=CC(=C1)NC(=O)NC2=CC=CC(=C2)C(=O)NC3=C4C(=CC(=CC4=C(C=C3)S(=O)(=O)[O-])S(=O)(=O)[O-])S(=O)(=O)[O-])C(=O)NC5=C6C(=CC(=CC6=C(C=C5)S(=O)(=O)[O-])S(=O)(=O)[O-])S(=O)(=O)[O-].[Na+].[Na+].[Na+].[Na+].[Na+].[Na+] | ||
| Standard InChIKey | FMQURVHYTBGYSQ-UHFFFAOYSA-H | ||
| Standard InChI | InChI=1S/C35H26N4O21S6.6Na/c40-33(38-25-7-9-27(63(49,50)51)23-13-21(61(43,44)45)15-29(31(23)25)65(55,56)57)17-3-1-5-19(11-17)36-35(42)37-20-6-2-4-18(12-20)34(41)39-26-8-10-28(64(52,53)54)24-14-22(62(46,47)48)16-30(32(24)26)66(58,59)60;;;;;;/h1-16H,(H,38,40)(H,39,41)(H2,36,37,42)(H,43,44,45)(H,46,47,48)(H,49,50,51)(H,52,53,54)(H,55,56,57)(H,58,59,60);;;;;;/q;6*+1/p-6 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | A subtype-selective, competitive and reversible P2X1 receptor antagonist. Displays IC50 values of 0.21, 28.9, > 50 and > 100 μM for human P2X1, P2X3, P2X2, and P2X4-mediated responses respectively. Selective over adrenoceptors, histamine and P2Y receptors. Also selectively inhibits the α-subunit of Go/i (EC50 ~300 nM). |

NF 023 Dilution Calculator

NF 023 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 0.8599 mL | 4.2997 mL | 8.5995 mL | 17.199 mL | 21.4987 mL |
| 5 mM | 0.172 mL | 0.8599 mL | 1.7199 mL | 3.4398 mL | 4.2997 mL |
| 10 mM | 0.086 mL | 0.43 mL | 0.8599 mL | 1.7199 mL | 2.1499 mL |
| 50 mM | 0.0172 mL | 0.086 mL | 0.172 mL | 0.344 mL | 0.43 mL |
| 100 mM | 0.0086 mL | 0.043 mL | 0.086 mL | 0.172 mL | 0.215 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- NF 157
Catalog No.:BCC7367
CAS No.:104869-26-3
- UPF 1069
Catalog No.:BCC2213
CAS No.:1048371-03-4
- Germanicol acetate
Catalog No.:BCN7264
CAS No.:10483-91-7
- Alpha-Terpineol
Catalog No.:BCN8136
CAS No.:10482-56-1
- COR 170
Catalog No.:BCC6282
CAS No.:1048039-15-1
- Masitinib mesylate
Catalog No.:BCC1729
CAS No.:1048007-93-7
- MRS 2768 tetrasodium salt
Catalog No.:BCC7800
CAS No.:1047980-83-5
- 4-O-Methylsappanol
Catalog No.:BCN5863
CAS No.:104778-16-7
- Sappanone B
Catalog No.:BCN7942
CAS No.:104778-15-6
- Plantamajoside
Catalog No.:BCN6279
CAS No.:104777-68-6
- 3alpha-Akebonoic acid
Catalog No.:BCN5862
CAS No.:104777-61-9
- Entacapone sodium salt
Catalog No.:BCC4107
CAS No.:1047659-02-8
- (+)-Isopulegol
Catalog No.:BCN4975
CAS No.:104870-56-6
- dl-Aloesol
Catalog No.:BCN7265
CAS No.:104871-04-7
- Borapetoside B
Catalog No.:BCN6593
CAS No.:104901-05-5
- Tranilast Sodium
Catalog No.:BCC4091
CAS No.:104931-56-8
- H-D-Val-OtBu.HCl
Catalog No.:BCC3146
CAS No.:104944-18-5
- Ethyl β-D-ribo-hex-3-ulopyranoside
Catalog No.:BCC8977
CAS No.:104953-08-4
- 8-Hydroxydigitoxigenin
Catalog No.:BCN5864
CAS No.:1049674-06-7
- Naspm trihydrochloride
Catalog No.:BCC7476
CAS No.:1049731-36-3
- A 331440 dihydrochloride
Catalog No.:BCC7963
CAS No.:1049740-32-0
- Cardiogenol C hydrochloride
Catalog No.:BCC7790
CAS No.:1049741-55-0
- PS 1145 dihydrochloride
Catalog No.:BCC7949
CAS No.:1049743-58-9
- 3-Acetoxy-4,7(11)-cadinadien-8-one
Catalog No.:BCN5865
CAS No.:104975-02-2
The effect of P2 receptor antagonists and ATPase inhibition on sympathetic purinergic neurotransmission in the guinea-pig isolated vas deferens.[Pubmed:10725256]
Br J Pharmacol. 2000 Mar;129(6):1089-94.
1. Intracellular microelectrodes were used to record the transmembrane potential and excitatory junction potentials (e.j.p.s) produced by sympathetic nerve stimulation (1 Hz) in smooth muscle cells of the guinea-pig isolated vas deferens. 2. The symmetrical 3'-urea of 8-(benzamido)naphthalene-1,3,5-trisulphonic acid (NF023) produced a concentration-dependent inhibition of e.j.p. magnitude (IC(50)=4. 8x10(-6) M), but had no effect on the resting membrane potential of the smooth muscle cells. 3. Pyridoxal-5-phosphate (P-5-P) also depressed e.j.p. magnitude in a concentration-dependent manner, but was less potent than NF023 (IC(50)=2.2x10(-5) M). At 10(-4) M and above P-5-P significantly depolarized the smooth muscle cells. 4. The nucleoside triphosphatase inhibitor 6-N,N-diethyl-D-beta, gamma-dibromomethyleneATP (ARL 67156) (5x10(-5) M) significantly increased e.j.p. amplitude. ARL 67156 (10(-4) M) further increased e. j.p. amplitude such that they often reached threshold for initiation of action potentials, causing muscle contraction and expulsion of the recording electrode. 5. After reduction of e.j.p.s by NF023 or P-5-P (both 10(-5) M), subsequent co-addition of ARL 67156 (10(-4) M) significantly increased their magnitude. 6. The overflow of endogenous ATP evoked by field stimulation of sympathetic nerves (8 Hz, 1 min) was measured by HPLC and flurometric detection. ARL 67156 (10(-4) M) enhanced ATP overflow by almost 700% compared to control. 7. We conclude that for electrophysiological studies NF023 is preferable to other P2X receptor antagonists such as pyridoxalphosphate -6-azophenyl-2',4'-disulphonic acid (PPADS), suramin or P-5-P. Furthermore, breakdown of endogenous ATP by nucleoside triphosphatases is an important modulator of purinergic neurotransmission in the guinea-pig vas deferens.
Antagonistic properties of the suramin analogue NF023 at heterologously expressed P2X receptors.[Pubmed:10193905]
Neuropharmacology. 1999 Jan;38(1):141-9.
The suramin analogue 8,8'-(carbonylbis(imino-3,1-phenylene carbonylimino)bis(1,3,5-naphthalenetrisulfonic acid) (NF023) antagonizes in a competitive fashion P2X receptor-mediated responses in certain vascular and visceral smooth muscles. In the present study, the effect of NF023 on voltage-clamped Xenopus oocytes heterologously expressing homomultimeric P2X1-P2X4 as well as heteromultimeric P2X2/P2X3 receptors has been characterized. P2X1 receptors were most sensitive to inhibition by NF023 with IC50 values of 0.24 and 0.21 microM for the rat and human homologue, respectively. P2X3 receptors have an intermediate sensitivity with IC50 values of 8.5 and 28.9 microM for rat and human subtypes, respectively and P2X2 was the least sensitive subtype (IC50 > 50 microM). P2X4 receptors were insensitive to NF023 at concentrations up to 100 microM. Coexpression of rat P2X3 with rat P2X2 resulted in receptors whose sensitivity to NF023 was identical to that obtained for homomultimeric rat P2X3 receptors (alphabeta meATP as agonist; IC50 = 1.4 and 1.6 microM, respectively). NF023 inhibited P2X1 receptors in a voltage-insensitive manner. In addition, NF023 (5 and 30 microM) caused a shift of the concentration-response curve to the right without affecting the maximal response to ATP (K(B) = 1.1 +/- 0.2 microM). Our results indicate that NF023 is a subtype-selective and surmountable antagonist at P2X1 receptors heterologously expressed in Xenopus oocytes.
Suramin analogues as subtype-selective G protein inhibitors.[Pubmed:8609887]
Mol Pharmacol. 1996 Apr;49(4):602-11.
G protein alpha subunits expose specific binding sites that allow for the sequential, conformation-dependent binding of protein reaction partners, e.g., G protein beta gamma dimers, receptors, and effectors. These domains represent potential sites for binding of low-molecular-weight inhibitors. We tested the following suramin analogues as G protein antagonists: 8-(3-nitrobenzamido)-1,3,5-naphtalenetrisulfonic acid (NF007), 8-(3-(3-nitrobenzamido)benzamido)-1,3,5-naphtalenetrisulfonic++ + acid NF018), 8,8'-(carbonylbis(imino-3,1-phenylene))bis-(1,3,5-naphtalenetri sulfonic acid) (NF023), 8,8'-(carbonylbis(imino-3,1-phenylene)carbonylimino-(3,1-phe nylene))bis-(1,3, 5-naphtalenetrisulfonic acid) (NF037), and suramin. The compounds suppressed [35S]GTPgammaS binding to purified, recombinant G protein alpha subunits, an effect that is due to inhibition of GDP release. Suramin is selective for recombinant Gsalpha-s (EC50 values o f approximately 240 nM; rank order of potency, suramin > NF037 > NF023 > NF018 > NF007), whereas NF023 is selective for recombinant Gi alpha-1 and recombinant Go alpha (EC50 value of approximately 300 nM; rank order of potency, NF023 > / = NF037 > suramin >0 NF018 > NF007). Selectivity was also demonstrated on a cellular level. In rat sympathetic neurons, alpha-2-adrenergic and muscarinic receptor-dependent inhibition of the voltage-sensitive calcium current is mediated by Gi/Go, whereas inhibition by vasoactive intestinal peptide (VIP) is mediated by Gs. Calcium current inhibition by alpha2-adrenergic and muscarinic receptors was greatly reduced when 100 microM NF023 was applied intracellularly, whereas the response to VIP was unaffected; in contrast, the response to VIP was blunted only with 100 microM suramin in the recording pipette. The suramin analogues do not interfere with the interaction between alpha subunits and G protein beta gamma dimer but compete with binding of the effector. The addition of purified adenylyl cyclase reverses the inhibitory effect of suramin on the rate of [35S]GTPgammaS binding to recombinant Gsalpha-s, indicating direct competition for a common site; similarly, immunoprecipitation by an antibody directed against an epitope of the effector binding site is inhibited by suramin. Our results show that it is possible to design G protein inhibitors that target the effector binding site on the alpha subunits.
Novel competitive antagonists for P2 purinoceptors.[Pubmed:7925607]
Eur J Pharmacol. 1994 Jun 15;268(1):1-7.
Binding of the radioligand [35S]adenosine 5'-O-(2-thiodiphosphate) (ADP beta 35S) to P2 gamma purinoceptors on turkey erythrocyte membranes was used to determine the affinity of suramin and various suramin congeners belonging to different structure classes (large urea, small urea, dibenzamides and benzamides) for these receptors. Suramin was shown to be a competitive antagonist with a Ki value of 7.3 +/- 2.2 microM. The simple benzamide compound XAMR0721 (8-(3,5-dinitrophenylene carbonylimino)-1,3,5-naphthalene trisulfonate, trisodium salt) displays a high affinity for the P2 gamma purinoceptor (Ki value of 19 +/- 6 microM). Similar to suramin, compound XAMR0721 is a competitive antagonist at P2 gamma purinoceptors. In contrast to suramin, which is a potent inhibitor of the ecto-nucleotidase activity in human blood cells (44 +/- 2% residual activity at 100 microM), compound XAMR0721 is hardly active in this assay (93 +/- 1% residual activity at 100 microM). So XAMR0721, the first competitive antagonist for P2 purinoceptors that is able to discriminate between P2 purinoceptor affinity and ecto-nucleotidase activity, is an interesting pharmacological tool for the characterization of P2 purinoceptor mediated effects.


