NBI-74330CXCR3 antagonist CAS# 855527-92-3 |
- Limonin
Catalog No.:BCN6057
CAS No.:1180-71-8
- Fosamprenavir Calcium Salt
Catalog No.:BCC1581
CAS No.:226700-81-8
- HIV-1 integrase inhibitor
Catalog No.:BCC1618
CAS No.:544467-07-4
- BMS-626529
Catalog No.:BCC1427
CAS No.:701213-36-7
- HIV-1 integrase inhibitor 2
Catalog No.:BCC1619
CAS No.:957890-42-5
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 855527-92-3 | SDF | Download SDF |
| PubChem ID | 45784923 | Appearance | Powder |
| Formula | C32H27F4N5O3 | M.Wt | 605.58 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 35 mg/mL (57.80 mM) *"≥" means soluble, but saturation unknown. | ||
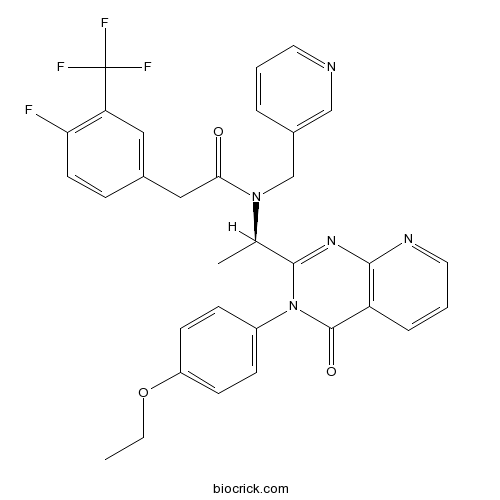
| Chemical Name | N-[(1R)-1-[3-(4-ethoxyphenyl)-4-oxopyrido[2,3-d]pyrimidin-2-yl]ethyl]-2-[4-fluoro-3-(trifluoromethyl)phenyl]-N-(pyridin-3-ylmethyl)acetamide | ||
| SMILES | CCOC1=CC=C(C=C1)N2C(=O)C3=C(N=CC=C3)N=C2C(C)N(CC4=CN=CC=C4)C(=O)CC5=CC(=C(C=C5)F)C(F)(F)F | ||
| Standard InChIKey | XMRGQUDUVGRCBS-HXUWFJFHSA-N | ||
| Standard InChI | InChI=1S/C32H27F4N5O3/c1-3-44-24-11-9-23(10-12-24)41-30(39-29-25(31(41)43)7-5-15-38-29)20(2)40(19-22-6-4-14-37-18-22)28(42)17-21-8-13-27(33)26(16-21)32(34,35)36/h4-16,18,20H,3,17,19H2,1-2H3/t20-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | NBI-74330 is a potent antagonist for CXCR3, and exhibits potent inhibition of (125I)CXCL10 and (125I)CXCL11 specific binding with Ki of 1.5 and 3.2 nM, respectively.In Vitro:BI-74330 demonstrates potent inhibition of [125I]CXCL11 specific binding to membranes prepared from transfected CHO cells expressing CXCR3 (CXCR3-CHO) (Ki=3.6 nM). NBI-74330 is 12- and 3.5-fold more potent than CXCL9 (Ki=45.2 nM) and CXCL10 (Ki=12.5 nM), respectively, at inhibiting [125I]CXCL11 binding to CXCR3-CHO cell membranes. NBI-74330 inhibits calcium mobilization in response to CXCL11 and CXCL10 with an IC50 value of 7 nM for both ligands used at their EC80 concentrations (1 nM for CXCL11 and 30 nM for CXCL10). NBI-74330 specifically inhibits CXCR3-mediated calcium mobilization. NBI-74330 also dose-dependently inhibits CXCL11-induced [35S]GTPγS binding in membranes of cells endogenously expressing CXCR3 (H9 cells, IC50 value 5.5 nM). BI-74330 inhibits CXCL11-induced chemotaxis in these cells with an IC50 of 3.9 nM[1]. NBI-74330 (30-300 nm, 1-10 μM) produces concentration-dependent, parallel rightward shifts of the CXCL11 E/[A] curve with no significant change in the E/[A] curve maximal response[2].In Vivo:NBI-74330 (100 mg/kg) results in the formation of an N-oxide metabolite, also an antagonist of CXCR3, in mice[2]. Mice treated with 100 mg/kg NBI-74330 (in 1% Na Doc in 0.5% 400Cp Methylcellulose) result in serum concentrations of approximately 1 μM. This concentration is sufficient to fully block the CXCR3 receptor in vivo[3]. References: | |||||

NBI-74330 Dilution Calculator

NBI-74330 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.6513 mL | 8.2565 mL | 16.5131 mL | 33.0262 mL | 41.2827 mL |
| 5 mM | 0.3303 mL | 1.6513 mL | 3.3026 mL | 6.6052 mL | 8.2565 mL |
| 10 mM | 0.1651 mL | 0.8257 mL | 1.6513 mL | 3.3026 mL | 4.1283 mL |
| 50 mM | 0.033 mL | 0.1651 mL | 0.3303 mL | 0.6605 mL | 0.8257 mL |
| 100 mM | 0.0165 mL | 0.0826 mL | 0.1651 mL | 0.3303 mL | 0.4128 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
NBI-74330 is an antagonist of CXC chemokine receptor 3 (CXCR3) with IC50 values of 7nM to18nM [1].
CXCR3 receptors are expressed on the activated Th1 cells and involved in inflammatory diseases. The three ligands of CXCR3 are CXCL9, CXCL10 and CXCL11. NBI-74330 is an antagonist of CXCR3 and is one of the T487 series which is developed for psoriasis and RA. In the competition binding assay, NBI-74330 is more potent than CXCL9 and CXCL10 at inhibiting CXCL11 binding to CXCR3. When treated with RBL cells, NBI-74330 inhibits calcium mobilization in response to CXCL11 and CXCL10 with IC50 values of 7nM for both. Besides that, NBI-74330 inhibits CXCL11-induced chemotaxis with IC50 of 3.9nM in H9 cells. NBI-74330 also inhibits the ligand-induced G protein activation. Moreover, NBI-74330 shows no significant inhibition of chemotaxis induced by the other chemokines such as CXCL12 and CCL19 [1].
References:
[1] Heise C E, Pahuja A, Hudson S C, et al. Pharmacological characterization of CXC chemokine receptor 3 ligands and a small molecule antagonist. Journal of Pharmacology and Experimental Therapeutics, 2005, 313(3): 1263-1271.
- Safflor Yellow A
Catalog No.:BCN2408
CAS No.:85532-77-0
- PK 11195
Catalog No.:BCC6745
CAS No.:85532-75-8
- Eupatorin
Catalog No.:BCN4405
CAS No.:855-96-9
- 4-Chlorotestosterone acetate
Catalog No.:BCC8705
CAS No.:855-19-6
- Ajugamarin chlorohydrin
Catalog No.:BCN3664
CAS No.:85447-27-4
- Caffeic anhydride
Catalog No.:BCN3295
CAS No.:854237-32-4
- (R)-(-)-Rolipram
Catalog No.:BCC5429
CAS No.:85416-75-7
- S- (+)-Rolipram
Catalog No.:BCC2303
CAS No.:85416-73-5
- Rilmenidine Phosphate
Catalog No.:BCC5637
CAS No.:85409-38-7
- Ropivacaine mesylate
Catalog No.:BCC9137
CAS No.:854056-07-8
- (-)-Haplomyrfolin
Catalog No.:BCN3225
CAS No.:85404-48-4
- Norcaesalpinin E
Catalog No.:BCN7006
CAS No.:854038-96-3
- Rhodionin
Catalog No.:BCN1248
CAS No.:85571-15-9
- Kurarinol
Catalog No.:BCN3447
CAS No.:855746-98-4
- Zaltidine
Catalog No.:BCC2068
CAS No.:85604-00-8
- Gynuramine
Catalog No.:BCN2085
CAS No.:85611-43-4
- (2-Aminoethyl)phosphinic acid
Catalog No.:BCN1761
CAS No.:85618-16-2
- Temozolomide
Catalog No.:BCC4386
CAS No.:85622-93-1
- WP1130
Catalog No.:BCC3686
CAS No.:856243-80-6
- Boc-Ala-NH2
Catalog No.:BCC3046
CAS No.:85642-13-3
- Curculigoside
Catalog No.:BCN4406
CAS No.:85643-19-2
- Laurycolactone A
Catalog No.:BCN3109
CAS No.:85643-76-1
- Laurycolactone B
Catalog No.:BCN3110
CAS No.:85643-77-2
- Mirtazapine
Catalog No.:BCC4923
CAS No.:85650-52-8
Analysis of the pharmacokinetic/pharmacodynamic relationship of a small molecule CXCR3 antagonist, NBI-74330, using a murine CXCR3 internalization assay.[Pubmed:17982480]
Br J Pharmacol. 2007 Dec;152(8):1260-71.
BACKGROUND AND PURPOSE: Pharmacokinetic/pharmacodynamic (PK/PD) models are necessary to relate the degree of drug exposure in vivo to target blockade and pharmacological efficacy. This manuscript describes a murine agonist-induced CXCR3 receptor internalization assay and demonstrates its utility for PK/PD analyses. EXPERIMENTAL APPROACH: Activated murine DO11.10 cells were incubated with agonist in the presence or absence of a CXCR3 antagonist and changes in surface CXCR3 expression were detected by flow cytometry. For PK/PD analysis, mice were dosed with a small molecule CXCR3 antagonist, NBI-74330, (100 mg kg(-1)) orally or subcutaneously and plasma samples taken at specified timepoints for the CXCR3 internalization assay. KEY RESULTS: Surface CXCR3 expression was specifically decreased in response to CXCL9, CXCL10 and CXCL11. CXCL11 was the most potent CXCR3 agonist in buffer (pA50=9.23+/-0.26) and the pA50 for CXCL11 was unaltered in murine plasma (pA50=9.17+/-0.15). The affinity of a small molecule CXCR3 antagonist, NBI-74330, was obtained in the absence or presence of plasma (buffer pA2 value: 7.84+/-0.14; plasma pKB) value 6.36+/-0.01). Administration of NBI-74330 to mice resulted in the formation of an N-oxide metabolite, also an antagonist of CXCR3. Both antagonists were detectable up to 7 h post oral dose and 24 h post subcutaneous dose. Measurement of CXCR3 internalization demonstrated significant antagonism of this response ex vivo, 24 h following subcutaneous administration of NBI-74330. CONCLUSIONS AND IMPLICATIONS: The CXCR3 receptor internalization assay provides a robust method for determining agonist potency orders, antagonist affinity estimates and PK/PD analyses, which discriminate between dosing regimens for the CXCR3 antagonist NBI-74330.
Identification of overlapping but differential binding sites for the high-affinity CXCR3 antagonists NBI-74330 and VUF11211.[Pubmed:24174496]
Mol Pharmacol. 2014 Jan;85(1):116-26.
CXC chemokine receptor CXCR3 and/or its main three ligands CXCL9, CXCL10, and CXCL11 are highly upregulated in a variety of diseases. As such, considerable efforts have been made to develop small-molecule receptor CXCR3 antagonists, yielding distinct chemical classes of antagonists blocking binding and/or function of CXCR3 chemokines. Although it is suggested that these compounds bind in an allosteric fashion, thus far no evidence has been provided regarding the molecular details of their interaction with CXCR3. Using site-directed mutagenesis complemented with in silico homology modeling, we report the binding modes of two high-affinity CXCR3 antagonists of distinct chemotypes: VUF11211 [(S)-5-chloro-6-(4-(1-(4-chlorobenzyl)piperidin-4-yl)-3-ethylpiperazin-1-yl)-N-et hylnicotinamide] (piperazinyl-piperidine) with a rigid elongated structure containing two basic groups and NBI-74330 [(R)-N-(1-(3-(4-ethoxyphenyl)-4-oxo-3,4-dihydropyrido[2,3-d]pyrimidin-2-yl)ethyl) -2-(4-fluoro-3-(trifluoromethyl)phenyl)-N-(pyridin-3-ylmethyl)acetamide] (8-azaquinazolinone) without any basic group. Here we show that NBI-74330 is anchored in the transmembrane minor pocket lined by helices 2 (W2.60, D2.63), 3 (F3.32), and 7 (S7.39, Y7.43), whereas VUF11211 extends from the minor pocket into the major pocket of the transmembrane domains, located between residues in helices 1 (Y1.39), 2 (W2.60), 3 (F3.32), 4 (D4.60), 6 (Y6.51), and 7 (S7.39, Y7.43). Mutation of these residues did not affect CXCL11 binding significantly, confirming the allosteric nature of the interaction of these small molecules with CXCR3. Moreover, the model derived from our in silico-guided studies fits well with the already published structure-activity relationship data on these ligands. Altogether, in this study, we show overlapping, yet different binding sites for two high-affinity CXCR3 antagonists, which offer new opportunities for the structure-based design of allosteric modulators for CXCR3.
CXCR3 antagonist NBI-74330 attenuates atherosclerotic plaque formation in LDL receptor-deficient mice.[Pubmed:18048768]
Arterioscler Thromb Vasc Biol. 2008 Feb;28(2):251-7.
OBJECTIVE: The chemokine receptor CXCR3 is implicated in migration of leukocytes to sites of inflammation. Antagonizing CXCR3 may be a strategy to inhibit inflammation-induced leukocyte migration and subsequently reduce atherosclerosis. We used the CXCR3 specific antagonist NBI-74330 to block CXCR3-mediated signaling in peritonitis and diet-induced atherosclerosis. METHODS AND RESULTS: Antagonizing CXCR3 with NBI-74330 resulted in a significant reduction in CD4+ T cell and macrophage migration to the peritoneal cavity, which was as shown in ex vivo migration studies totally CXCR3 dependent. Atherosclerotic lesion formation in the aortic valve leaflet area and the entire aorta was significantly inhibited in NBI-74330 treated mice. Lymph nodes draining from the aortic arch were significantly smaller in treated mice and were enriched in regulatory T cells and contained fewer activated T cells, whereas the markers for regulatory T cells within the lesion were enhanced after NBI-74330 treatment. CONCLUSIONS: This study shows for the first time that treatment with a CXCR3 antagonist results in attenuating atherosclerotic lesion formation by blocking direct migration of CXCR3+ effector cells from the circulation into the atherosclerotic plaque and by beneficially modulating the inflammatory response in the lesion and the lymph nodes draining from the atherosclerotic lesion.


