MetforminCAS# 657-24-9 |
Quality Control & MSDS
Number of papers citing our products

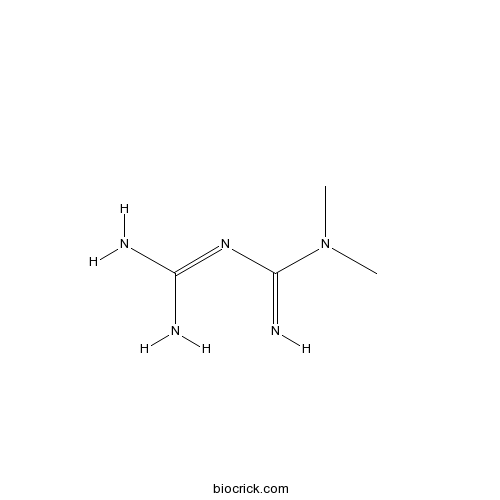
Chemical structure

3D structure
| Cas No. | 657-24-9 | SDF | Download SDF |
| PubChem ID | 4091 | Appearance | Powder |
| Formula | C4H11N5 | M.Wt | 129 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 3-(diaminomethylidene)-1,1-dimethylguanidine | ||
| SMILES | CN(C)C(=N)N=C(N)N | ||
| Standard InChIKey | XZWYZXLIPXDOLR-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C4H11N5/c1-9(2)4(7)8-3(5)6/h1-2H3,(H5,5,6,7,8) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Metformin Dilution Calculator

Metformin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 7.7519 mL | 38.7597 mL | 77.5194 mL | 155.0388 mL | 193.7984 mL |
| 5 mM | 1.5504 mL | 7.7519 mL | 15.5039 mL | 31.0078 mL | 38.7597 mL |
| 10 mM | 0.7752 mL | 3.876 mL | 7.7519 mL | 15.5039 mL | 19.3798 mL |
| 50 mM | 0.155 mL | 0.7752 mL | 1.5504 mL | 3.1008 mL | 3.876 mL |
| 100 mM | 0.0775 mL | 0.3876 mL | 0.7752 mL | 1.5504 mL | 1.938 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Reversine
Catalog No.:BCC1892
CAS No.:656820-32-5
- TC-C 14G
Catalog No.:BCC6144
CAS No.:656804-72-7
- AG-1024
Catalog No.:BCC1242
CAS No.:65678-07-1
- HA14-1
Catalog No.:BCC3593
CAS No.:65673-63-4
- Silybin B maltoside
Catalog No.:BCC8250
CAS No.:335299-49-5
- Esculentoside E
Catalog No.:BCN5014
CAS No.:65649-36-7
- Fenretinide
Catalog No.:BCC1572
CAS No.:65646-68-6
- Nintedanib (BIBF 1120)
Catalog No.:BCC3661
CAS No.:656247-17-5
- Ophiopogonin D'
Catalog No.:BCN2645
CAS No.:65604-80-0
- Cerberic acid
Catalog No.:BCN4200
CAS No.:65597-44-6
- Cerbinal
Catalog No.:BCN4199
CAS No.:65597-42-4
- 4'-Demethylepipodophyllotoxin
Catalog No.:BCN5918
CAS No.:6559-91-7
- H-Lys-OH.2HCl
Catalog No.:BCC2979
CAS No.:657-26-1
- H-Lys-OH.HCl
Catalog No.:BCC2978
CAS No.:657-27-2
- Z-D-Glu-OBzl
Catalog No.:BCC2774
CAS No.:65706-99-2
- Apramycin Sulfate
Catalog No.:BCC4628
CAS No.:65710-07-8
- Boc-Dap(Z)-OH.DCHA
Catalog No.:BCC2667
CAS No.:65710-58-9
- Z-D-Ser(tBu)-OH
Catalog No.:BCC2739
CAS No.:65806-90-8
- 3-Eudesmene-1beta,11-diol
Catalog No.:BCN7096
CAS No.:658062-22-7
- 10(14)-Cadinene-4,11-diol
Catalog No.:BCN7099
CAS No.:658062-23-8
- Goserelin Acetate
Catalog No.:BCC5352
CAS No.:65807-02-5
- SU11274
Catalog No.:BCC1243
CAS No.:658084-23-2
- FK866 (APO866)
Catalog No.:BCC2332
CAS No.:658084-64-1
- Peucedanol 7-O-glucoside
Catalog No.:BCN7689
CAS No.:65853-04-5
Metformin reduces prostate cancer risk among men with benign prostatic hyperplasia: A nationwide population-based cohort study.[Pubmed:30968600]
Cancer Med. 2019 Apr 9.
Benign Prostate Hyperplasia (BPH) has been associated with prostate cancer prevalent among men after 50 years of age, however, it is unclear whether the antidiabetic drug, Metformin, can reduce prostate cancer for men with BPH. The insurance claims data of men aged 50 years or older, with both type 2 diabetes mellitus (T2DM) and BPH diagnosed from 1997 to 2007 were analyzed. Individuals were followed up for at least 5 years. We identified 2906 and 2906 patients as the Metformin cohort and nonMetformin cohort, respectively. The Cox method analysis showed that the Metformin cohort had an adjusted hazard ratio (aHR) of 0.69 (95% confidence interval [CI] = 0.49-0.96, P = 0.0298) for prostate cancer, compared to the nonMetformin cohort after controlling for age, traditional Chinese medicine (TCM) use, prostate specific antigen, and Charlson comorbidity index. Patients using TCM for BPH (per 6 months) also had an aHR of 0.41 (95% CI = 0.24-0.69; P = 0.0009). In conclusion, both Metformin medication and TCM use could be associated with reduced risk of prostate cancer for men with BPH and diabetes.
Lactic acidosis due to metformin in type 2 diabetes mellitus and chronic kidney disease stage 3-5: is it significant?[Pubmed:30963454]
Int Urol Nephrol. 2019 Apr 8. pii: 10.1007/s11255-019-02136-y.
PURPOSE: To study the incidence of lactic acidosis due to Metformin in patients with type 2 diabetes mellitus (T2DM) and chronic kidney disease (CKD) stage 3-5. METHODS: We estimated plasma lactate in patients of CKD stage 3 and worse who were continuing Metformin on their own prior to stopping the drug. RESULT: Of 40 patients included, median duration of T2DM was 60 months (interquartile range IQR 24-120). The mean serum creatinine was 309.4 +/- 159.1 micromol/L and mean eGFR was 27.82 +/- 12.93 mL/min/1.73 m(2) with 3 (7.5%), 16 (40%), 11 (27.5%) and 10 (25%) in CKD stages 3a, 3b, 4 and 5, respectively. They were receiving Metformin for a median duration of 24 months (IQR 12.5-60), an average dose of 896 +/- 350 mg per day. The median of plasma lactate was 1.36 mmol/L (IQR 1.11-1.75 mmol/L) with three (7.5%) having levels above normal, two (20%) in CKD stage 5 and one (9.1%) in stage 4. CONCLUSION: Metformin can be safely used in CKD stage 3 and with regular measurement of plasma lactate in later stages.
Association between Polymorphisms of OCT1 and Metabolic Response to Metformin in Women with Polycystic Ovary Syndrome.[Pubmed:30959948]
Int J Mol Sci. 2019 Apr 7;20(7). pii: ijms20071720.
Insulin-sensitizer treatment with Metformin is widely used in polycystic ovary syndrome (PCOS). However, the treatment effectiveness shows individual differences in PCOS patients. Organic cation transporter (OCT) 1 and 2 have been reported to mediate Metformin transport in the liver and kidney, respectively. In this study, we investigated the association between the polymorphisms of OCT1 and OCT2 and the treatment effectiveness of Metformin in PCOS patients. The single nucleotide polymorphisms (SNPs) of OCT1 (rs683369 and rs628031) and OCT2 (rs316019) were analyzed in 87 PCOS and 113 control women. Oral glucose tolerance tests (OGTTs), which represented Metformin treatment response, were conducted at the start of treatment and after six-month treatment. The results demonstrated that the SNP frequencies of OCT1 and OCT2 were not associated with PCOS pathophysiology, and that the polymorphisms of OCT1 and OCT2 were not associated with the OGTT parameters at baseline. However, PCOS patients with the G allele of OCT1 rs683369 and/or with the A allele of OCT1 rs628031 had increased insulin sensitivity compared to those with wild-type genotype after receiving Metformin treatment. Moreover, the interactions of Metformin*SNP were significant in both OCT1 rs683369 (p < 0.001) and rs628031 (p = 0.001) during the treatment period. Taken together, genetic polymorphisms of OCT1 contributed to different Metformin treatment responses, and further study is needed to establish personalized treatment programs using a pharmacogenomic algorithm approach in PCOS patients.
The Potential Use of Metformin, Dipyridamole, N-Acetylcysteine and Statins as Adjunctive Therapy for Systemic Lupus Erythematosus.[Pubmed:30959892]
Cells. 2019 Apr 6;8(4). pii: cells8040323.
Systemic lupus erythematosus (SLE) is a chronic inflammatory autoimmune condition that can potentially affect every single organ during the course of the disease, leading to increased morbidity and mortality, and reduced health-related quality of life. While curative treatment is currently non-existent for SLE, therapeutic agents such as glucocorticoids, mycophenolate, azathioprine, cyclosporine, cyclophosphamide and various biologics are the mainstay of treatment based on their immunomodulatory and immunosuppressive properties. As a result of global immunosuppression, the side-effect profile of the current therapeutic approach is unfavourable, with adverse effects including myelosuppression, infection and malignancies. Hydroxychloroquine, one of the very few Food and Drug Administration (FDA)-approved medications for the treatment of SLE, has been shown to offer a number of therapeutic benefits to SLE patients independent of its immunomodulatory effect. As such, it is worth exploring drugs similar to hydroxychloroquine that confer additional clinical benefits unrelated to immunosuppressive mechanisms. Indeed, apart from hydroxychloroquine, a number of studies have explored the use of a few conventionally non-immunosuppressive drugs that are potentially useful in the management of SLE. In this review, non-immunosuppressive therapeutic agents, namely Metformin, dipyridamole, N-acetylcysteine and statins, will be critically discussed with regard to their mechanisms of action and efficacy pertaining to their potential therapeutic role in SLE.
Metformin treatment affects adipocytokine secretion and lipid composition in adipose tissues of diet-induced insulin-resistant rats.[Pubmed:30959381]
Nutrition. 2019 Jan 30;63-64:126-133.
OBJECTIVES: Adipose tissue plays a central role in the pathogenesis of insulin resistance (IR) and type 2 diabetes. However, the molecular changes that promote these diseases are not completely understood. Several studies demonstrated that ceramide (Cer) and diacylglycerol (DAG) accumulation in muscle is associated with IR. The aim of this study was to explain whether a high-fat diet (HFD) leads to bioactive lipid accumulation in adipose tissue and how Metformin affects the lipid content in adipocytes and the concentration of plasma adipocytokines. METHODS: The experiments were conducted on male Wistar rats divided into three groups: control, HFD-fed, and HFD-fed and treated with Metformin. Cer and DAGs were analyzed by liquid chromatography tandem mass spectrometry. Phosphorylation of hormone-sensitive lipase (HSL) was analyzed by Western blot. Oral glucose tolerance and insulin tolerance tests were also performed. Plasma adiponectin and tumor necrosis factor (TNF)-alpha concentration were measured by enzyme-linked immunosorbent assay. RESULTS: HFD induced IR and elevated DAGs and Cer content in subcutaneous and visceral adipose tissues, which was accompanied by an increased phosphorylation of HSL. Metformin improved insulin sensitivity, decreased Cer and DAG levels, and attenuated the phosphorylation of HSL in both fat depots. Furthermore, we observed a strong correlation between adiponectin (negative) and TNF-alpha (positive) and bioactive lipids in both fat tissues. CONCLUSIONS: These results indicated that bioactive lipids accumulation in adipose tissue influences the induction of IR and, at least in part, answered the question of what the insulin-sensitizing effect of Metformin at the level of adipose tissue is.
Metformin Improves Learning and Memory in the SAMP8 Mouse Model of Alzheimer's Disease.[Pubmed:30958364]
J Alzheimers Dis. 2019 Apr 3. pii: JAD181240.
Metformin is used for the treatment of insulin resistant diabetes. Diabetics are at an increased risk of developing dementia. Recent epidemiological studies suggest that Metformin treatment prevents cognitive decline in diabetics. A pilot clinical study found cognitive improvement with Metformin in patients with mild cognitive impairment (MCI). Preclinical studies suggest Metformin reduces Alzheimer-like pathology in mouse models of Alzheimer's disease (AD). In the current study, we used 11-month-old SAMP8 mice. Mice were given daily injections of Metformin at 20 mg/kg/sc or 200 mg/kg/sc for eight weeks. After four weeks, mice were tested in T-maze footshock avoidance, object recognition, and Barnes maze. At the end of the study, brain tissue was collected for analysis of PKC (PKCzeta, PKCiota, PKCalpha, PKCgamma, PKCvarepsilon), GSK-3beta, pGSK-3betaser9, pGSK-3betatyr216, pTau404, and APP. Metformin improved both acquisition and retention in SAMP8 mice in T-maze footshock avoidance, retention in novel object recognition, and acquisition in the Barnes maze. Biochemical analysis indicated that Metformin increased both atypical and conventional forms of PKC; PKCzeta, and PKCalpha at 20 mg/kg. Metformin significantly increased pGSK-3betaser9 at 200 mg/kg, and decreased Abeta at 20 mg/kg and pTau404 and APPc99 at both 20 mg/kg and 200 mg/kg. There were no differences in blood glucose levels between the aged vehicle and Metformin treated mice. Metformin improved learning and memory in the SAMP8 mouse model of spontaneous onset AD. Biochemical analysis indicates that Metformin improved memory by decreasing APPc99 and pTau. The current study lends support to the therapeutic potential of Metformin for AD.


