LY 233053CAS# 125546-04-5 |
- T0901317
Catalog No.:BCC1178
CAS No.:293754-55-9
- GW3965
Catalog No.:BCC1612
CAS No.:405911-09-3
- GW3965 HCl
Catalog No.:BCC3790
CAS No.:405911-17-3
- Fexaramine
Catalog No.:BCC7412
CAS No.:574013-66-4
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 125546-04-5 | SDF | Download SDF |
| PubChem ID | 3035974 | Appearance | Powder |
| Formula | C8H13N5O2 | M.Wt | 211.22 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in water and to 100 mM in DMSO | ||
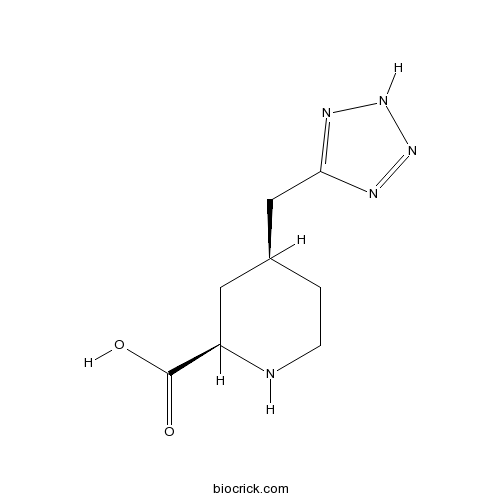
| Chemical Name | (2R,4S)-4-(2H-tetrazol-5-ylmethyl)piperidine-2-carboxylic acid | ||
| SMILES | C1CNC(CC1CC2=NNN=N2)C(=O)O | ||
| Standard InChIKey | FAAVTENFCLADRE-NTSWFWBYSA-N | ||
| Standard InChI | InChI=1S/C8H13N5O2/c14-8(15)6-3-5(1-2-9-6)4-7-10-12-13-11-7/h5-6,9H,1-4H2,(H,14,15)(H,10,11,12,13)/t5-,6+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Competitive NMDA receptor antagonist (IC50 = 7 nM) that displays no affinity for AMPA or kainate receptors at a concentration of 10 μM. Inhibits NMDA-induced neuronal degeneration and protects from NMDA-induced convulsions in neonatal rats. |

LY 233053 Dilution Calculator

LY 233053 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.7344 mL | 23.672 mL | 47.344 mL | 94.688 mL | 118.36 mL |
| 5 mM | 0.9469 mL | 4.7344 mL | 9.4688 mL | 18.9376 mL | 23.672 mL |
| 10 mM | 0.4734 mL | 2.3672 mL | 4.7344 mL | 9.4688 mL | 11.836 mL |
| 50 mM | 0.0947 mL | 0.4734 mL | 0.9469 mL | 1.8938 mL | 2.3672 mL |
| 100 mM | 0.0473 mL | 0.2367 mL | 0.4734 mL | 0.9469 mL | 1.1836 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 3',5,5',7-Tetrahydroxy-4',6-dimethoxyflavone
Catalog No.:BCN6136
CAS No.:125537-92-0
- Dracorhodin perchlorate
Catalog No.:BCN2628
CAS No.:125536-25-6
- Testosterone phenylpropionate
Catalog No.:BCC9171
CAS No.:1255-49-8
- SR 8278
Catalog No.:BCC6191
CAS No.:1254944-66-5
- Sibutramine hydrochloride monohydrate
Catalog No.:BCC5251
CAS No.:125494-59-9
- Saclofen
Catalog No.:BCC6580
CAS No.:125464-42-8
- LY2874455
Catalog No.:BCC1723
CAS No.:1254473-64-7
- RQ-00203078
Catalog No.:BCC6419
CAS No.:1254205-52-1
- Acetate gossypol
Catalog No.:BCN5354
CAS No.:12542-36-8
- TCN 238
Catalog No.:BCC7901
CAS No.:125404-04-8
- Cedrelone
Catalog No.:BCN6135
CAS No.:1254-85-9
- 6-Acetonyl-N-methyl-dihydrodecarine
Catalog No.:BCN6134
CAS No.:1253740-09-8
- PF-4708671
Catalog No.:BCC5031
CAS No.:1255517-76-0
- UNC0638
Catalog No.:BCC1135
CAS No.:1255580-76-7
- MnTMPyP Pentachloride
Catalog No.:BCC6532
CAS No.:125565-45-9
- Rotigotine hydrochloride
Catalog No.:BCC1908
CAS No.:125572-93-2
- [Leu31,Pro34]-Neuropeptide Y (porcine)
Catalog No.:BCC5716
CAS No.:125580-28-1
- TRX818
Catalog No.:BCC6458
CAS No.:1256037-58-7
- AI-10-49
Catalog No.:BCC3973
CAS No.:1256094-72-0
- RuBi-Nicotine
Catalog No.:BCC7793
CAS No.:1256362-30-7
- Ledipasvir
Catalog No.:BCC1696
CAS No.:1256388-51-8
- CH5424802
Catalog No.:BCC3749
CAS No.:1256580-46-7
- Blinin
Catalog No.:BCN8455
CAS No.:125675-09-4
- 3''-O-acetyl-platyconic acid A
Catalog No.:BCN3319
CAS No.:1256935-28-0
Competitive NMDA-receptor antagonists, LY 235959 and LY 233053, enhance the protective efficacy of various antiepileptic drugs against maximal electroshock-induced seizures in mice.[Pubmed:8681893]
Epilepsia. 1996 Jul;37(7):618-24.
PURPOSE: The objective of this study was to evaluate an interaction of two competitive N-methyl-D-aspartate (NMDA)-receptor antagonists, LY 235959 l(-)-3R,4aS,6R,8aR-6-(phosphonomethyl)-decahydroiso-qu inoline-3-carboxylic acid; < or = 0.5 mg/kg] or LY 233053 cis-(+/-)-4-[(2H-tetrazol-5-yl) methyl]piperidine-2-carboxylic acid; < or = 5 mg/kg] with carbamazepine, diphenylhydantoin, phenobarbital, or valproate magnesium against maximal electroshock-induced convulsions in mice. METHODS: Electroconvulsions were produced by means of an alternating current (ear-clip electrodes, 0.2-s stimulus duration, tonic hindlimb extension taken as the end point) delivered by a Hugo-Sachs stimulator (Type 221, reiburg, FRG). Adverse effects were evaluated in the chimney test (motor performance) and passive-avoidance ask (long-term memory). Plasma levels of antiepileptic rugs were measured by immunofluorescence. RESULTS: Both LY 235959 and LY 233053 ( < or = 0.5 and 5 mg/kg, respectively) did not influence the electroconvulsive threshold but potentiated the anticonvulsant action of all antiepileptics studied. The combined treatment of LY 233053 (5 mg/kg) with carbamazepine, diphenylhydantoin, or phenobarbital (providing a 50% protection against maximal electroshock) resulted in the impairment of long-term memory. No adverse effects were observed with combinations of LY 235959 with these antiepileptics. The combined treatment of valproate with either LY 235959 or LY 233053 was superior to valproate alone, as regards motor impairment, but not the impairment of long-term memory. Neither NMDA-receptor antagonist elevated the total plasma levels of antiepileptic drugs studied. CONCLUSIONS: It may be concluded that NMDA-receptor blockade leads to the enhanced anticonvulsive action of conventional antiepileptics against maximal electroshock-induced seizures. A pharmacokinetic interaction does not seem probable.
Delayed therapy of experimental ischemia with competitive N-methyl-D-aspartate antagonists in rabbits.[Pubmed:8322381]
Stroke. 1993 Jul;24(7):1068-71.
BACKGROUND AND PURPOSE: N-methyl-D-aspartate antagonists are effective in limiting ischemic damage to the brain and spinal cord if treatment is begun at time of ischemic injury. More clinically relevant delayed therapy has not been adequately investigated. We report the temporal profile of efficacy for two competitive N-methyl-D-aspartate antagonists in therapy of central nervous system ischemia. METHODS: CGS-19755 (30 mg/kg) or LY233053 (100 mg/kg) was administered 5, 30, or 60 minutes after reversible spinal cord ischemia in rabbits, induced by temporary occlusion of the infrarenal aorta. Duration of occlusion for individual animals was varied to provide a range of ischemia for each experimental group. The P50 represents the duration (in minutes) associated with a 50% probability of resultant permanent paraplegia. Neuroprotection is demonstrated if a drug prolongs the P50. RESULTS: CGS-19755 significantly prolonged the P50 (t test, P = .003) when given 5 minutes after ischemia, but not if delayed by 30 or 60 minutes (P50: control, 24.1; 5 minutes, 31.4; 30 minutes, 30.1; 60 minutes, 26.6). LY233053 was efficacious at 5 (P = .0008) and 30 (P = .002) minutes, but not at 60 minutes (P50: control, 26.8; 5 minutes, 39.4; 30 minutes, 36.0; 60 minutes, 25.6). CONCLUSIONS: These competitive N-methyl-D-aspartate antagonists are effective in limiting ischemic damage, but protection is lost if therapy is not initiated within 60 minutes of injury.
Efficacy of LY233053, a competitive glutamate antagonist, in experimental central nervous system ischemia.[Pubmed:1727148]
J Neurosurg. 1992 Jan;76(1):106-10.
Antagonists of excitatory amino acids appear to serve a neuroprotective role during ischemic conditions in a variety of in vivo and in vitro models. The usefulness of such agents in the clinical setting, however, may be limited by poor central nervous system (CNS) entry and intolerable side effects. The authors report high efficacy in reducing neurological damage and relatively limited side effects of LY233053, a novel competitive glutamate antagonist, in two models of experimental CNS ischemia in the rabbit.
Pharmacological characterization of LY233053: a structurally novel tetrazole-substituted competitive N-methyl-D-aspartic acid antagonist with a short duration of action.[Pubmed:2148188]
J Pharmacol Exp Ther. 1990 Dec;255(3):1301-8.
This study reports the activity of a structurally novel excitatory amino acid receptor antagonist, LY233053 [cis-(+-)-4-[(2H-tetrazol-5-yl)methyl]piperidine-2-carboxylic acid], the first tetrazole-containing competitive N-methyl-D-aspartic acid (NMDA) antagonist. LY233053 potently inhibited NMDA receptor binding to rat brain membranes as shown by the in vitro displacement of [3H] CGS19755 (IC50 = 107 +/- 7 nM). No appreciable affinity in [3H]alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) or [3H]kainate binding assays was observed (IC50 values greater than 10,000 nM). In vitro NMDA receptor antagonist activity was further demonstrated by selective inhibition of NMDA-induced depolarization in cortical wedges (IC50 = 4.2 +/- 0.4 microM vs. 40 microM NMDA). LY233053 was effective after in vivo systemic administration in a number of animal models. In neonatal rats, LY233053 selectively blocked NMDA-induced convulsions (ED50 = 14.5 mg/kg i.p.) with a relatively short duration of action (2-4 hr). In pigeons, LY233053 potently antagonized (ED50 = 1.3 mg/kg i.m.) the behavioral suppressant effects of 10 mg/kg of NMDA. However, a dose of 160 mg/kg, i.m., was required to produce phencyclidine-like catalepsy in pigeons. In mice, LY233053 protected against maximal electroshock-induced seizures at lower doses (ED50 = 19.9 mg/kg i.p.) than those that impaired horizontal screen performance (ED50 = 40.9 mg/kg i.p.). Cholinergic and GABAergic neuronal degenerations after striatal infusion of NMDA were prevented by single or multiple i.p. doses of LY233053. In summary, the antagonist activity of LY233053 after systemic administration demonstrates potential therapeutic value in conditions of neuronal cell loss due to NMDA receptor excitotoxicity. The relatively short duration of action of LY233053 may make this compound particularly advantageous as a neuroprotective agent in the treatment of acute conditions such as cerebral ischemia.


