L803-mtsSelective inhibitor of GSK-3 CAS# 1043881-55-5 |
- Dexpramipexole dihydrochloride
Catalog No.:BCC1528
CAS No.:104632-27-1
- Dexpramipexole
Catalog No.:BCC1527
CAS No.:104632-28-2
- Cariprazine hydrochloride
Catalog No.:BCC1454
CAS No.:1083076-69-0
- Cariprazine
Catalog No.:BCC1453
CAS No.:839712-12-8
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 1043881-55-5 | SDF | Download SDF |
| PubChem ID | 90488753 | Appearance | Powder |
| Formula | C66H110N15O20P | M.Wt | 1464.64 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 1 mg/ml in 20% acetonitrile / water | ||
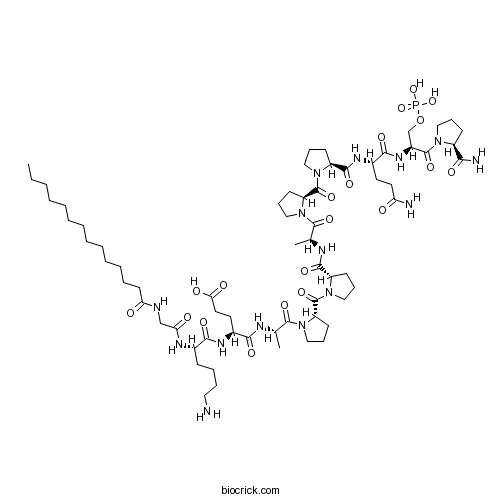
| Sequence | GKEAPPAPPQSP (Modifications: Gly-1 = Myr-Gly, Pro-12 = C-terminal amide, Ser-11 = pSer) | ||
| Chemical Name | (4S)-5-[[(2S)-1-[(2S)-2-[(2S)-2-[[(2S)-1-[(2S)-2-[(2S)-2-[[(2S)-5-amino-1-[[(2S)-1-[(2S)-2-carbamoylpyrrolidin-1-yl]-1-oxo-3-phosphonooxypropan-2-yl]amino]-1,5-dioxopentan-2-yl]carbamoyl]pyrrolidine-1-carbonyl]pyrrolidin-1-yl]-1-oxopropan-2-yl]carbamoyl]pyrrolidine-1-carbonyl]pyrrolidin-1-yl]-1-oxopropan-2-yl]amino]-4-[[(2S)-6-amino-2-[[2-(tetradecanoylamino)acetyl]amino]hexanoyl]amino]-5-oxopentanoic acid | ||
| SMILES | CCCCCCCCCCCCCC(=O)NCC(=O)NC(CCCCN)C(=O)NC(CCC(=O)O)C(=O)NC(C)C(=O)N1CCCC1C(=O)N2CCCC2C(=O)NC(C)C(=O)N3CCCC3C(=O)N4CCCC4C(=O)NC(CCC(=O)N)C(=O)NC(COP(=O)(O)O)C(=O)N5CCCC5C(=O)N | ||
| Standard InChIKey | JLHWBVQBEGDSEZ-LFOOZZFTSA-N | ||
| Standard InChI | InChI=1S/C66H110N15O20P/c1-4-5-6-7-8-9-10-11-12-13-14-28-53(83)70-39-54(84)73-43(22-15-16-33-67)58(89)74-45(30-32-55(85)86)57(88)71-41(2)62(93)80-37-20-26-50(80)65(96)78-35-18-24-48(78)60(91)72-42(3)63(94)81-38-21-27-51(81)66(97)79-36-19-25-49(79)61(92)75-44(29-31-52(68)82)59(90)76-46(40-101-102(98,99)100)64(95)77-34-17-23-47(77)56(69)87/h41-51H,4-40,67H2,1-3H3,(H2,68,82)(H2,69,87)(H,70,83)(H,71,88)(H,72,91)(H,73,84)(H,74,89)(H,75,92)(H,76,90)(H,85,86)(H2,98,99,100)/t41-,42-,43-,44-,45-,46-,47-,48-,49-,50-,51-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective peptide inhibitor of glycogen synthase kinase-3 (GSK-3). Produces antidepressive behavior in mice when administered in vivo. Shows no inhibition of PKC, PKB or cdc2 protein kinase. Exhibits insulin mimetic activity. Activates glycogen synthase activity 2.5-fold in HEK293 cells. Myristoylated version of L803. |

L803-mts Dilution Calculator

L803-mts Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- RU-SKI 43
Catalog No.:BCC5441
CAS No.:1043797-53-0
- Tetrahydroxysqualene
Catalog No.:BCN5858
CAS No.:1043629-23-7
- Bisoprolol fumarate
Catalog No.:BCC4344
CAS No.:104344-23-2
- IRAK inhibitor 6
Catalog No.:BCC1658
CAS No.:1042672-97-8
- Famciclovir
Catalog No.:BCC4780
CAS No.:104227-87-4
- IRAK inhibitor 1
Catalog No.:BCC1654
CAS No.:1042224-63-4
- Yunnandaphninine G
Catalog No.:BCN5857
CAS No.:1042143-83-8
- Estriol 3,17-dihexanoate
Catalog No.:BCN2238
CAS No.:104202-96-2
- 10-Nitro-camptothecin
Catalog No.:BCN2581
CAS No.:104195-61-1
- Stanozolol
Catalog No.:BCC9154
CAS No.:10418-03-8
- Alpinoid D
Catalog No.:BCN3593
CAS No.:1041740-13-9
- MitoPY1
Catalog No.:BCC6177
CAS No.:1041634-69-8
- Typhaneoside
Catalog No.:BCN4994
CAS No.:104472-68-6
- 3'-Methylflavokawin
Catalog No.:BCN3990
CAS No.:1044743-35-2
- RVX-208
Catalog No.:BCC4475
CAS No.:1044870-39-4
- Testosterone acetate
Catalog No.:BCC9165
CAS No.:1045-69-8
- Bayogenin 3-O-beta-D-glucopyranoside
Catalog No.:BCN7868
CAS No.:104513-86-2
- EC 23
Catalog No.:BCC6097
CAS No.:104561-41-3
- CAY10603
Catalog No.:BCC5542
CAS No.:1045792-66-2
- p-Menthan-3-one
Catalog No.:BCN3850
CAS No.:10458-14-7
- Caffeic acid phenethyl ester
Catalog No.:BCN2695
CAS No.:104594-70-9
- CGS 15943
Catalog No.:BCC7157
CAS No.:104615-18-1
- Pramipexole dihydrochloride
Catalog No.:BCN2181
CAS No.:104632-25-9
- Pramipexole
Catalog No.:BCC4467
CAS No.:104632-26-0
The inhibitor of glycerol 3-phosphate acyltransferase FSG67 blunts liver regeneration after acetaminophen overdose by altering GSK3beta and Wnt/beta-catenin signaling.[Pubmed:30654094]
Food Chem Toxicol. 2019 Mar;125:279-288.
Repair mechanisms after acetaminophen (APAP) hepatotoxicity are poorly understood. We recently discovered that phosphatidic acid (PA) increases in mice and humans after APAP overdose, and is critical for liver regeneration. Here, we hypothesized that PA inhibits glycogen synthase kinase-3beta (GSK3beta), a component of canonical Wnt/beta-catenin signaling, after APAP overdose. To test that, we treated mice with 300mg/kg APAP at 0h followed by vehicle or 20mg/kg of the glycerol 3-phosphate acyltransferase inhibitor FSG67 at 3, 24 and 48h. Some mice also received the GSK3 inhibitor L803-mts. Blood and liver were collected at multiple time points. Consistent with our earlier results, FSG67 did not affect toxicity (ALT, histology), APAP bioactivation (total glutathione), or oxidative stress (oxidized glutathione), but did reduce expression of proliferating cell nuclear antigen (PCNA) at 52h. We then measured GSK3beta phosphorylation and found it was dramatically decreased by FSG67 at 24h, before PCNA dropped. Expression of cyclin D1, downstream of Wnt/beta-catenin, was also reduced. To determine if the effect of FSG67 on GSK3beta is important, we treated mice with FSG67 and L803-mts after APAP. Importantly, L803-mts rescued hepatocyte proliferation and survival. Our data indicate PA and lysoPA may support recovery after APAP overdose by inhibiting GSK3beta.
Inhibition of Glycogen Synthase Kinase 3 Accelerated Liver Regeneration after Acetaminophen-Induced Hepatotoxicity in Mice.[Pubmed:28068511]
Am J Pathol. 2017 Mar;187(3):543-552.
Overdose of acetaminophen (APAP) is the leading cause of acute liver failure (ALF) in the United States. Timely initiation of compensatory liver regeneration after APAP hepatotoxicity is critical for final recovery, but the mechanisms of liver regeneration after APAP-induced ALF have not been extensively explored yet. Previous studies from our laboratory have demonstrated that activation of beta-catenin signaling after APAP overdose is associated with timely liver regeneration. Herein, we investigated the role of glycogen synthase kinase 3 (GSK3) in liver regeneration after APAP hepatotoxicity using a pharmacological inhibition strategy in mice. Treatment with specific GSK3 inhibitor (L803-mts), starting from 4 hours after 600 mg/kg dose of APAP, resulted in early initiation of liver regeneration in a dose-dependent manner, without modifying the peak regenerative response. Acceleration of liver regeneration was not secondary to alteration of APAP-induced hepatotoxicity, which remained unchanged after GSK3 inhibition. Early cell cycle initiation in hepatocytes after GSK3 inhibition was because of rapid induction of cyclin D1 and phosphorylation of retinoblastoma protein. This was associated with increased activation of beta-catenin signaling after GSK3 inhibition. Taken together, our study has revealed a novel role of GSK3 in liver regeneration after APAP overdose and identified GSK3 as a potential therapeutic target to improve liver regeneration after APAP-induced ALF.
Ketamine up-regulates a cluster of intronic miRNAs within the serotonin receptor 2C gene by inhibiting glycogen synthase kinase-3.[Pubmed:27723376]
World J Biol Psychiatry. 2017 Sep;18(6):445-456.
OBJECTIVES: We examined mechanisms that contribute to the rapid antidepressant effect of ketamine in mice that is dependent on glycogen synthase kinase-3 (GSK3) inhibition. METHODS: We measured serotonergic (5HT)-2C-receptor (5HTR2C) cluster microRNA (miRNA) levels in mouse hippocampus after administering an antidepressant dose of ketamine (10 mg/kg) in wild-type and GSK3 knockin mice, after GSK3 inhibition with L803-mts, and in learned helpless mice. RESULTS: Ketamine up-regulated cluster miRNAs 448-3p, 764-5p, 1264-3p, 1298-5p and 1912-3p (2- to 11-fold). This up-regulation was abolished in GSK3 knockin mice that express mutant constitutively active GSK3. The GSK3 specific inhibitor L803-mts was antidepressant in the learned helplessness and novelty suppressed feeding depression-like behaviours and up-regulated the 5HTR2C miRNA cluster in mouse hippocampus. After administration of the learned helplessness paradigm mice were divided into cohorts that were resilient (non-depressed) or were susceptible (depressed) to learned helplessness. The resilient, but not depressed, mice displayed increased hippocampal levels of miRNAs 448-3p and 1264-3p. Administration of an antagonist to miRNA 448-3p diminished the antidepressant effect of ketamine in the learned helplessness paradigm, indicating that up-regulation of miRNA 448-3p provides an antidepressant action. CONCLUSIONS: These findings identify a new outcome of GSK3 inhibition by ketamine that may contribute to antidepressant effects.
Up-regulation of insulin-like growth factor 2 by ketamine requires glycogen synthase kinase-3 inhibition.[Pubmed:27542584]
Prog Neuropsychopharmacol Biol Psychiatry. 2017 Jan 4;72:49-54.
An antidepressant dose of the rapidly-acting ketamine inhibits glycogen synthase kinase-3 (GSK3) in mouse hippocampus, and this inhibition is required for the antidepressant effect of ketamine in learned helplessness depression-like behavior. Here we report that treatment with an antidepressant dose of ketamine (10mg/kg) increased expression of insulin-like growth factor 2 (IGF2) in mouse hippocampus, an effect that required ketamine-induced inhibition of GSK3. Ketamine also inhibited hippocampal GSK3 and increased expression of hippocampal IGF2 in mice when administered after the induction of learned helplessness. Treatment with the specific GSK3 inhibitor L803-mts was sufficient to up-regulate hippocampal IGF2 expression. Administration of IGF2 siRNA reduced ketamine's antidepressant effect in the learned helplessness paradigm. Mice subjected to the learned helplessness paradigm were separated into two groups, those that were resilient (non-depressed) and those that were susceptible (depressed). Non-depressed resilient mice displayed higher expression of IGF2 than susceptible mice. These results indicate that IGF2 contributes to ketamine's antidepressant effect and that IGF2 may confer resilience to depression-like behavior.
GSK-3beta controls autophagy by modulating LKB1-AMPK pathway in prostate cancer cells.[Pubmed:26440826]
Prostate. 2016 Feb;76(2):172-83.
BACKGROUND: Glycogen synthase kinase 3beta (GSK3B, GSK-3beta) is a multi-functional protein kinase involved in various cellular processes and its activity elevates after serum deprivation. We have shown that inhibition of GSK-3beta activity triggered a profound autophagic response and subsequent necrotic cell death after serum deprivation in prostate cancer cells. In this study, we dissected the mechanisms involved in GSK-3beta inhibition-triggered autophagy. METHODS: Prostate cancer PC-3 and DU145 cells were used in the study. Multiple GSK-3beta specific inhibitors were used including small chemicals TDZD8, Tideglusib, TWS119, and peptide L803-mts. Western blot assay coupled with phospho-specific antibodies were used in detecting signal pathway activation. ATP levels were assessed with ATPLite kit and HPLC methods. Autophagy response was determined by evaluating Microtubule-associated proteins 1A/1B light chain 3B (LC3B) processing and p62 protein stability in Western blot assays. Immunofluorescent microscopy was used to detect LKB1 translocation. RESULTS: Inhibition of GSK-3beta activity resulted in a significant decline of cellular ATP production, leading to a significant increase of AMP/ATP ratio, a strong trigger of AMP-activated protein kinase (AMPK) activation in prostate cancer PC-3 cells. In parallel with increased LC-3B biosynthesis and p62 protein reduction, the classical sign of autophagy induction, AMPK was activated after inhibition of GSK-3beta activity. Further analysis revealed that Liver kinase B1 (LKB1) but not Calcium/calmodulin-dependent protein kinase kinase beta (CaMKKbeta) is involved in AMPK activation and autophagy induction triggered by GSK-3beta inhibition. Meanwhile, GSK-3beta inhibition promoted LKB1 translocation from nuclear to cytoplasmic compartment and enhanced LKB1 interaction with its regulatory partners Mouse protein-25 (MO25) and STE20-related adaptor (STRAD). CONCLUSIONS: In conclusion, our data suggest that GSK-3beta plays an important role in controlling autophagy induction by modulating the activation of LKB1-AMPK pathway after serum deprivation.
GSK-3 and lysosomes meet in Alzheimer's disease.[Pubmed:23940827]
Commun Integr Biol. 2013 Sep 1;6(5):e25179. Epub 2013 Jun 4.
Aberrant regulation of glycogen synthase kinase-3 (GSK-3) is implicated in Alzheimer's disease (AD), but the mechanisms involved remain elusive. Our recent study shows that GSK-3 impairs lysosomal acidification and that inhibition of GSK-3 re-acidified lysosomes in brains of AD mice. This effect was accompanied by reductions in beta-amyloid pathology and amelioration of cognitive deficits. Presenilin-1 (PS1) is an essential factor in lysosomal acidification. To determine whether the inhibition of GSK-3 restores lysosomal malfunction caused by dysfunctional PS1, we treated MEF cells deficient in presenilin proteins (MEF-PS1/2(-/-)) with a selective substrate competitive GSK-3 inhibitor, L803-mts. L803-mts enhanced the acidic lysosomal pool in MEF-PS1/2(-/-) cells and increased levels of activated cathepsin D in the lysosomes. We conclude that GSK-3 and PS1 operate via similar mechanisms to disrupt lysosomal acidification. Importantly, these data indicate that GSK-3 inhibitors have potential in treatment of conditions associated with defective PS1.
Regulation of Th1 cells and experimental autoimmune encephalomyelitis by glycogen synthase kinase-3.[Pubmed:23606540]
J Immunol. 2013 May 15;190(10):5000-11.
Experimental autoimmune encephalomyelitis (EAE) is a rodent model of multiple sclerosis (MS), a debilitating autoimmune disease of the CNS, for which only limited therapeutic interventions are available. Because MS is mediated in part by autoreactive T cells, particularly Th17 and Th1 cells, in the current study, we tested whether inhibitors of glycogen synthase kinase-3 (GSK3), previously reported to reduce Th17 cell generation, also alter Th1 cell production or alleviate EAE. GSK3 inhibitors were found to impede the production of Th1 cells by reducing STAT1 activation. Molecularly reducing the expression of either of the two GSK3 isoforms demonstrated that Th17 cell production was sensitive to reduced levels of GSK3beta and Th1 cell production was inhibited in GSK3alpha-deficient cells. Administration of the selective GSK3 inhibitors TDZD-8, VP2.51, VP0.7, or L803-mts significantly reduced the clinical symptoms of myelin oligodendrocyte glycoprotein35-55-induced EAE in mice, nearly eliminating the chronic progressive phase, and reduced the number of Th17 and Th1 cells in the spinal cord. Administration of TDZD-8 or L803-mts after the initial disease episode alleviated clinical symptoms in a relapsing-remitting model of proteolipid protein139-151-induced EAE. Furthermore, deletion of GSK3beta specifically in T cells was sufficient to alleviate myelin oligodendrocyte glycoprotein35-55-induced EAE. These results demonstrate the isoform-selective effects of GSK3 on T cell generation and the therapeutic effects of GSK3 inhibitors in EAE, as well as showing that GSK3 inhibition in T cells is sufficient to reduce the severity of EAE, suggesting that GSK3 may be a feasible target for developing new therapeutic interventions for MS.
Targeting of CRMP-2 to the primary cilium is modulated by GSK-3beta.[Pubmed:23185275]
PLoS One. 2012;7(11):e48773.
CRMP-2 plays a pivotal role in promoting axon formation, neurite outgrowth and elongation in neuronal cells. CRMP-2's role in other cells is unknown. Our preliminary results showed CRMP-2 expression in cilia of fibroblasts. To localize CRMP-2, define its role and study the regulation of CRMP-2's expression in cilia we carried out the following experiments. We find that in fibroblasts CRMP-2 localizes to the centrosome and is associated with the basal body and -at a low level- is present in primary cilia. Phosphorylated pCRMP-2 can only be detected at the basal body. RNAi knockdown of CRMP-2 interfered with primary cilium assembly demonstrating a critical requirement for CRMP-2. Deletion analysis of CRMP-2 identified a 51 amino acid sequence in the C-terminus that is required for targeting to the basal body and primary cilium. This domain contains GSK-3beta phosphorylation sites as well as two repeats of the VxPx motif, previously identified as a cilium targeting signal in other primary cilium proteins. To our surprise, mutation of the CRMP-2 VxPx motifs did not eliminate primary cilium targeting. Instead, mutation of the GSK-3beta phosphorylation sites abolished CRMP-2 targeting to the primary cilium without affecting basal body localization. Treatment of cells with lithium, a potent GSK-3beta inhibitor, or with two specific GSK-3beta inhibitors (the L803-mts peptide inhibitor and CHIR99021) resulted in cilium elongation and decreased basal body levels of pCRMP-2 as well as increased levels of total CRMP-2 at the primary cilium. In summary, we identified CRMP-2 as a protein critically involved in primary cilia formation. To our knowledge this is the first demonstration of modulation of primary cilium targeting by GSK-3beta.
Inhibition of glycogen synthase kinase-3 ameliorates beta-amyloid pathology and restores lysosomal acidification and mammalian target of rapamycin activity in the Alzheimer disease mouse model: in vivo and in vitro studies.[Pubmed:23155049]
J Biol Chem. 2013 Jan 11;288(2):1295-306.
Accumulation of beta-amyloid (Abeta) deposits is a primary pathological feature of Alzheimer disease that is correlated with neurotoxicity and cognitive decline. The role of glycogen synthase kinase-3 (GSK-3) in Alzheimer disease pathogenesis has been debated. To study the role of GSK-3 in Abeta pathology, we used 5XFAD mice co-expressing mutated amyloid precursor protein and presenilin-1 that develop massive cerebral Abeta loads. Both GSK-3 isozymes (alpha/beta) were hyperactive in this model. Nasal treatment of 5XFAD mice with a novel substrate competitive GSK-3 inhibitor, L803-mts, reduced Abeta deposits and ameliorated cognitive deficits. Analyses of 5XFAD hemi-brain samples indicated that L803-mts restored the activity of mammalian target of rapamycin (mTOR) and inhibited autophagy. Lysosomal acidification was impaired in the 5XFAD brains as indicated by reduced cathepsin D activity and decreased N-glycoyslation of the vacuolar ATPase subunit V0a1, a modification required for lysosomal acidification. Treatment with L803-mts restored lysosomal acidification in 5XFAD brains. Studies in SH-SY5Y cells confirmed that GSK-3alpha and GSK-3beta impair lysosomal acidification and that treatment with L803-mts enhanced the acidic lysosomal pool as demonstrated in LysoTracker Red-stained cells. Furthermore, L803-mts restored impaired lysosomal acidification caused by dysfunctional presenilin-1. We provide evidence that mTOR is a target activated by GSK-3 but inhibited by impaired lysosomal acidification and elevation in amyloid precursor protein/Abeta loads. Taken together, our data indicate that GSK-3 is a player in Abeta pathology. Inhibition of GSK-3 restores lysosomal acidification that in turn enables clearance of Abeta burdens and reactivation of mTOR. These changes facilitate amelioration in cognitive function.
Suppression of glycogen synthase kinase 3 activity reduces tumor growth of prostate cancer in vivo.[Pubmed:21456066]
Prostate. 2011 Jun 1;71(8):835-45.
BACKGROUND: Glycogen synthase kinase 3 (GSK-3) has been regarded as a potential therapeutic target for multiple human cancers. We previously reported that suppression of GSK-3 activity with lithium chloride (LiCl) or small chemical inhibitors impaired cellular DNA synthesis and reduced cell proliferation in prostate cancer cells. Therefore, in this study, we extended this in vitro findings to in vivo settings in order to establish a proof of concept that inhibition of GSK-3 activity is feasible in suppressing tumor growth of prostate cancer in vivo. METHODS: In this study, we used three GSK-3 inhibitors, LiCl, TDZD-8, and L803-mts, which are structurally unrelated and non-ATP competitive. Human prostate cancer cell lines PC-3 and C4-2 were used for nude mouse xenograft models. The autochthonous transgenic prostate cancer TRAMP mice were used for testing GSK-3 inhibitor's effect on tumor development. Anti-Ki-67 and BrdU immunohistochemistry was used to determine cell proliferation. The pE2F-TA-LUC (E2F-LUC) luciferase reporter assay and gene specific small interferencing RNA technique were used to examine C/EBP involvement in GSK-3 inhibitor-induced E2F-1 suppression. RESULTS: Using mouse xenograft models, we demonstrated that LiCl and TDZD-8 significantly suppressed tumor development and growth of subcutaneous xenografts derived from human prostate cancer cells. Similarly, in the TRAMP mice, TDZD-8 and L803-mts reduced the incidence and tumor burden in the prostate lobes. Consistent with our previous in vitro findings, GSK-3 inhibitors significantly reduced BrdU incorporation and Ki67-positive cells in xenograft tumors and mouse cancerous prostates compared to the control. Further analysis revealed that following GSK-3 inhibition, C/EBPalpha, a negative cell cycle regulator, was remarkably accumulated in xenograft tumors or in cultured prostate cancer cells. Meanwhile, knocking down C/EBPalpha expression abolished GSK-3 inhibition-induced suppression of E2F1 transactivation, suggesting that C/EBPalpha accumulation is involved in GSK-3 inhibition-induced anti-tumor effect. CONCLUSION: Taken together, these results suggest that GSK-3 inhibition has the potential as a therapeutic strategy for prostate cancer intervention, although further pre-clinical and clinical testing are desirable.
Elucidating substrate and inhibitor binding sites on the surface of GSK-3beta and the refinement of a competitive inhibitor.[Pubmed:21354422]
J Mol Biol. 2011 Apr 29;408(2):366-78.
A molecular understanding of substrate recognition of protein kinases provides an important basis for the development of substrate competitive inhibitors. Here, we explored substrate recognition and competitive inhibition of glycogen synthase kinase (GSK)-3beta using molecular and computational tools. In previous work, we described Gln89 and Asn95 within GSK-3beta as important substrates binding sites. Here, we show that the cavity bordered by loop 89-QDKRFKN-95, located in the vicinity of the GSK-3beta catalytic core, is a promiscuous substrate binding subsite. Mutations within this segment highlighted Phe93 as an additional essential contact residue for substrates' recognition. However, unlike Gln89 and Asn95, Phe93 was also important for the binding of our previously described substrate competitive inhibitor, L803 [KEAPPAPPQS(p)P], and its cell-permeable variant L803-mts. The effects of the substitution of charged or polar residues within L803 further suggested that binding to GSK-3beta is governed by hydrophobic interactions. Our computational model of GSK-3beta bound to L803 was in agreement with the experimental data. It revealed L803 binding with a hydrophobic surface patch and identified interactions between Pro8 (L803) and Phe93 (GSK-3beta). Computational modeling of new L803 variants predicted that inhibition would be strengthened by adding contacts with Phe93 or by increasing the hydrophobic content of the peptide. Indeed, the newly designed L803 variants showed improved inhibition. Our study identified different and overlapping elements in GSK-3beta substrate and inhibitor recognition and provides a novel example for model-based rational design of substrate competitive inhibitors for GSK-3.
Role of Glycogen Synthase Kinase-3beta in APP Hyperphosphorylation Induced by NMDA Stimulation in Cortical Neurons.[Pubmed:27713242]
Pharmaceuticals (Basel). 2010 Jan 7;3(1):42-58.
The phosphorylation of Amyloid Precursor Protein (APP) at Thr(668) plays a key role in APP metabolism that is highly relevant to AD. The c-Jun-N-terminal kinase (JNK), glycogen synthase kinase-3beta (GSK-3beta) and cyclin-dependent kinase 5 (Cdk5) can all be responsible for this phosphorylation. These kinases are activated by excitotoxic stimuli fundamental hallmarks of AD. The exposure of cortical neurons to a high dose of NMDA (100 muM) for 30'-45' led to an increase of P-APP Thr(668). During NMDA stimulation APP hyperphosphorylation has to be assigned to GSK-3beta activity, since addition of L803-mts, a substrate competitive inhibitor of GSK-3beta reduced APP phosphorylation induced by NMDA. On the contrary, inhibition of JNK and Cdk5 with D-JNKI1 and Roscovitine respectively did not prevent NMDA-induced P-APP increase. These data show a tight connection, in excitotoxic conditions, between APP metabolism and the GSK-3beta signaling pathway.
Glycogen synthase kinase 3beta (GSK3beta) mediates 6-hydroxydopamine-induced neuronal death.[Pubmed:15132987]
FASEB J. 2004 Jul;18(10):1162-4.
The causes of sporadic Parkinson's disease (PD) are poorly understood. 6-Hydroxydopamine (6-OHDA), a PD mimetic, is widely used to model this neurodegenerative disorder in vitro and in vivo; however, the underlying mechanisms remain incompletely elucidated. We demonstrate here that 6-OHDA evoked endoplasmic reticulum (ER) stress, which was characterized by an up-regulation in the expression of GRP78 and GADD153 (Chop), cleavage of procaspase-12, and phosphorylation of eukaryotic initiation factor-2 alpha in a human dopaminergic neuronal cell line (SH-SY5Y) and cultured rat cerebellar granule neurons (CGNs). Glycogen synthase kinase-3 beta (GSK3beta) responds to ER stress, and its activity is regulated by phosphorylation. 6-OHDA significantly inhibited phosphorylation of GSK3beta at Ser9, whereas it induced hyperphosphorylation of Tyr216 with little effect on GSK3beta expression in SH-SY5Y cells and PC12 cells (a rat dopamine cell line), as well as CGNs. Furthermore, 6-OHDA decreased the expression of cyclin D1, a substrate of GSK3beta, and dephosphorylated Akt, the upstream signaling component of GSK3beta. Protein phosphatase 2A (PP2A), an ER stress-responsive phosphatase, was involved in 6-OHDA-induced GSK3beta dephosphorylation (Ser9). Blocking GSK3beta activity by selective inhibitors (lithium, TDZD-8, and L803-mts) prevented 6-OHDA-induced cleavage of caspase-3 and poly(ADP-ribose) polymerase (PARP), DNA fragmentations and cell death. With a tetracycline (Tet)-controlled TrkB inducible system, we demonstrated that activation of TrkB in SH-SY5Y cells alleviated 6-OHDA-induced GSK3beta dephosphorylation (Ser9) and ameliorated 6-OHDA neurotoxicity. TrkB activation also protected CGNs against 6-OHDA-induced damage. Although antioxidants also offered neuroprotection, they had little effect on 6-OHDA-induced GSK3beta activation. These results suggest that GSK3beta is a critical intermediate in pro-apoptotic signaling cascades that are associated with neurodegenerative diseases, thus providing a potential target site amenable to pharmacological intervention.
Rapid antidepressive-like activity of specific glycogen synthase kinase-3 inhibitor and its effect on beta-catenin in mouse hippocampus.[Pubmed:15050857]
Biol Psychiatry. 2004 Apr 15;55(8):781-4.
BACKGROUND: Inhibition of glycogen synthase kinase-3 (GSK-3) is thought to be a key feature in the therapeutic mechanism of several mood stabilizers; however, the role of GSK-3 in depressive behavior has not been determined. In these studies, we evaluated the antidepressive effect of L803-mts, a novel GSK-3 peptide inhibitor, in an animal model of depression, the mouse forced swimming test (FST). METHODS: Animals were intracerebroventricularly injected with L803-mts or with respective control peptide (cp) 1 hour, 3 hours, or 12 hours before their subjection to FST. RESULTS: Animals administered L803-mts showed reduced duration of immobility at all three time points tested, as compared with cp-treated animals. Expression levels of beta-catenin, the endogenous substrate of GSK-3, increased in the hippocampus of L803-mts-treated animals by 20%-50%, as compared with cp-treated animals. CONCLUSIONS: Our studies show, for the first time, that in-vivo inhibition of GSK-3 produces antidepressive-like behavior and suggest the potential of GSK-3 inhibitors as antidepressants.


