K145SphK2 inhibitor CAS# 1309444-75-4 |
- SB 431542
Catalog No.:BCC3658
CAS No.:301836-41-9
- SB-505124 hydrochloride
Catalog No.:BCC1930
CAS No.:356559-13-2
- SB525334
Catalog No.:BCC2531
CAS No.:356559-20-1
- A 77-01
Catalog No.:BCC1318
CAS No.:607737-87-1
- LY2109761
Catalog No.:BCC3806
CAS No.:700874-71-1
- LY2157299
Catalog No.:BCC3709
CAS No.:700874-72-2
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 1309444-75-4 | SDF | Download SDF |
| PubChem ID | 71714682 | Appearance | Powder |
| Formula | C18H24N2O3S | M.Wt | 348.46 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | SphK2 inhibitor | ||
| Solubility | Soluble in DMSO | ||
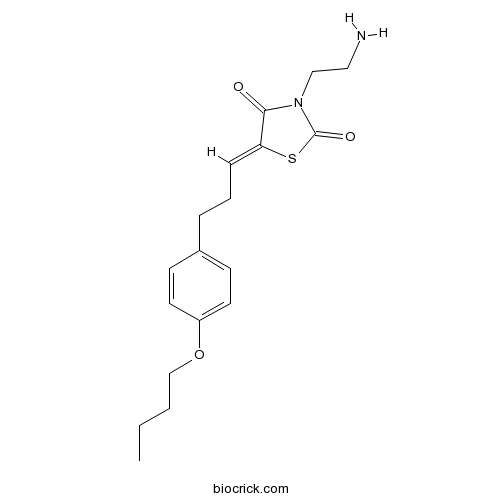
| Chemical Name | (5Z)-3-(2-aminoethyl)-5-[3-(4-butoxyphenyl)propylidene]-1,3-thiazolidine-2,4-dione | ||
| SMILES | CCCCOC1=CC=C(C=C1)CCC=C2C(=O)N(C(=O)S2)CCN | ||
| Standard InChIKey | MPZXLTZVPUSTFY-SOFYXZRVSA-N | ||
| Standard InChI | InChI=1S/C18H24N2O3S/c1-2-3-13-23-15-9-7-14(8-10-15)5-4-6-16-17(21)20(12-11-19)18(22)24-16/h6-10H,2-5,11-13,19H2,1H3/b16-6- | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | K145 is a selective SphK2 inhibitor with an IC50 of 4.30±0.06 μM , while no inhibition of SphK1 at concentrations up to 10 μM.
IC50 value: 4.3 uM [1]
Target: SphK2
in vitro: K145 inhibited the activity of SphK2 in a dose-dependent manner with an IC50 of 4.30±0.06 uM , while no inhibition of SphK1 at concentrations up to 10 uM was observed. Lineweaver-Burk analysis revealed a Ki of 6.4±0.7 uM for SphK2 and indicated that K145 is a substrate competitive inhibitor (with sphingosine). K145 accumulates in U937 cells, suppresses the S1P level, and inhibits SphK2. K145 also exhibited inhibitory effects on the growth of U937 cells as well as apoptotic effects in U937 cells, and that these effects may be through the inhibition of down-stream ERK and Akt signaling pathways [1].
in vivo: K145 also significantly inhibited the growth of U937 tumors in nude mice by both intraperitoneal and oral administration, thus demonstrating its in vivo efficacy as a potential lead anticancer agent [2]. References: | |||||

K145 Dilution Calculator

K145 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8698 mL | 14.3488 mL | 28.6977 mL | 57.3954 mL | 71.7442 mL |
| 5 mM | 0.574 mL | 2.8698 mL | 5.7395 mL | 11.4791 mL | 14.3488 mL |
| 10 mM | 0.287 mL | 1.4349 mL | 2.8698 mL | 5.7395 mL | 7.1744 mL |
| 50 mM | 0.0574 mL | 0.287 mL | 0.574 mL | 1.1479 mL | 1.4349 mL |
| 100 mM | 0.0287 mL | 0.1435 mL | 0.287 mL | 0.574 mL | 0.7174 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
K145 is a selective inhibitor of Sphingosine Kinase-2 (SphK2) with IC50 value of 4.3μM [1].
K145 is an analogue of sphingosine. It competes with the substrate of SphK2 and shows Ki value of 6.4μM in the in vitro assay. K145 is selective against SphK2 over SphK1 and CERK. In U937 cells, K145 decreases total cellular S1P and inhibits the phosphorylation of FTY720 (Both S1P and FTY720 are substrates of SphK2). Meanwhile, K145 suppresses cell growth and induces late apoptosis significantly. It is also found to inhibit the phosphorylation of ERK and Akt in this cell line [1].
In the U937 xenograft model in mice, K145 inhibits tumor growth with a TGI value of 44.2% and has less toxicity than tamibarotene. In BALB/c mice bearing the JC xenograft, K145 also shows potent inhibitory effects on tumor growth. Moreover, in BALB/c-nu mice bearing U937 xenograft, oral administration of K145 exerts better antitumor activity than tamibarotene [1].
References:
[1] Liu K, Guo T L, Hait N C, et al. Biological characterization of 3-(2-amino-ethyl)-5-[3-(4-butoxyl-phenyl)-propylidene]-thiazolidine-2, 4-dione (K145) as a selective sphingosine kinase-2 inhibitor and anticancer agent. PloS one, 2013, 8(2): e56471.
- GPR40 Activator 1
Catalog No.:BCC4125
CAS No.:1309435-60-6
- Cerberic acid B
Catalog No.:BCN4715
CAS No.:1309362-77-3
- CX-4945 sodium salt
Catalog No.:BCC5586
CAS No.:1309357-15-0
- Entacapone
Catalog No.:BCC2217
CAS No.:130929-57-6
- 7-O-Acetylneocaesalpin N
Catalog No.:BCN7332
CAS No.:1309079-08-0
- N4-Benzoylcytidine
Catalog No.:BCC9072
CAS No.:13089-48-0
- Fmoc-Val-OSu
Catalog No.:BCC3572
CAS No.:130878-68-1
- 1beta,10beta-Epoxydehydroleucodin
Catalog No.:BCN7331
CAS No.:130858-00-3
- ent-kaurane-3,16,17-triol
Catalog No.:BCN6164
CAS No.:130855-22-0
- Decursinol angelate
Catalog No.:BCC9222
CAS No.:130848-06-5
- Scoparinol
Catalog No.:BCN6163
CAS No.:130838-00-5
- 7-Oxohinokinin
Catalog No.:BCN6162
CAS No.:130837-92-2
- Wittifuran X
Catalog No.:BCN4794
CAS No.:1309478-07-6
- Marumoside A
Catalog No.:BCN7702
CAS No.:1309604-34-9
- H 89 2HCl
Catalog No.:BCC4997
CAS No.:130964-39-5
- (±)-CPSI 1306
Catalog No.:BCC6161
CAS No.:1309793-47-2
- Sephin1
Catalog No.:BCC3980
CAS No.:13098-73-2
- MRS 4062 triethylammonium salt
Catalog No.:BCC6134
CAS No.:1309871-50-8
- 15-Methoxymkapwanin
Catalog No.:BCN6498
CAS No.:1309920-99-7
- VU 0360172 hydrochloride
Catalog No.:BCC6141
CAS No.:1309976-62-2
- alpha-Yohimbine
Catalog No.:BCN6166
CAS No.:131-03-3
- Dimethyl phthalate
Catalog No.:BCN6167
CAS No.:131-11-3
- Pimpinellin
Catalog No.:BCN6168
CAS No.:131-12-4
- N-Acetylneuraminic acid
Catalog No.:BCN2204
CAS No.:131-48-6
Interactions of acetylcholine binding site residues contributing to nicotinic acetylcholine receptor gating: role of residues Y93, Y190, K145 and D200.[Pubmed:23831994]
J Mol Graph Model. 2013 Jul;44:145-54.
The nicotinic acetylcholine receptor exhibits multiple conformational states, resting (channel closed), active (channel open) and desensitized (channel closed). The resting state may be distinguished from the active and desensitized states by the orientation of loop C in the extracellular ligand binding domain (LBD). Homology modeling was used to generate structures of the Torpedo californica alpha2betadeltagamma nAChR that initially represent the resting state (loop C open) and the desensitized state (loop C closed). Molecular dynamics (MD) simulations were performed on the extracellular LBD on each nAChR conformational state, with and without the agonist anabaseine present in each binding site (the alphagamma and the alphadelta sites). Three MD simulations of 10ns each were performed for each of the four conditions. Comparison of dynamics revealed that in the presence of agonist, loop C was drawn inward and attains a more stable conformation. Examination of side-chain interactions revealed that residue alphaY190 exhibited hydrogen-bonding interactions either with residue alphaY93 in the ligand binding site or with residue alphaK145 proximal to the binding site. alphaK145 also exhibited side chain (salt bridge) interactions with alphaD200 and main chain interactions with alphaY93. Residues alphaW149, alphaY198, gammaY116/deltaT119, gammaL118/deltaL121 and gammaL108/deltaL111 appear to play the role of stabilizing ligand in the binding site. In MD simulations for the desensitized state, the effect of ligand upon the interactions among alphaK145, alphaY190, and alphaY93 as well as ligand-hydrogen-bonding to alphaW149 were more pronounced at the alphagamma interface than at the alphadelta interface. Differences in affinity for the desensitized state were determined experimentally to be 10-fold. The changes in side chain interactions observed for the two conformations and induced by ligand support a model wherein hydrogen bond interactions between alphaD200 and alphaY93 are broken and rearrange to form a salt-bridge between alphaK145 and alphaD200 and hydrogen bond interactions between alphaY93 and alphaY190 and between alphaK145 and alphaY190.
Biological characterization of 3-(2-amino-ethyl)-5-[3-(4-butoxyl-phenyl)-propylidene]-thiazolidine-2,4-dione (K145) as a selective sphingosine kinase-2 inhibitor and anticancer agent.[Pubmed:23437140]
PLoS One. 2013;8(2):e56471.
In our effort to develop selective sphingosine kinase-2 (SphK2) inhibitors as pharmacological tools, a thiazolidine-2,4-dione analogue, 3-(2-amino-ethyl)-5-[3-(4-butoxyl-phenyl)-propylidene]-thiazolidine-2,4-dione (K145), was synthesized and biologically characterized. Biochemical assay results indicate that K145 is a selective SphK2 inhibitor. Molecular modeling studies also support this notion. In vitro studies using human leukemia U937 cells demonstrated that K145 accumulates in U937 cells, suppresses the S1P level, and inhibits SphK2. K145 also exhibited inhibitory effects on the growth of U937 cells as well as apoptotic effects in U937 cells, and that these effects may be through the inhibition of down-stream ERK and Akt signaling pathways. K145 also significantly inhibited the growth of U937 tumors in nude mice by both intraperitoneal and oral administration, thus demonstrating its in vivo efficacy as a potential lead anticancer agent. The antitumor activity of K145 was also confirmed in a syngeneic mouse model by implanting murine breast cancer JC cells in BALB/c mice. Collectively, these results strongly encourage further optimization of K145 as a novel lead compound for development of more potent and selective SphK2 inhibitors.


