HarringtonolideCAS# 64761-48-4 |
Quality Control & MSDS
Number of papers citing our products

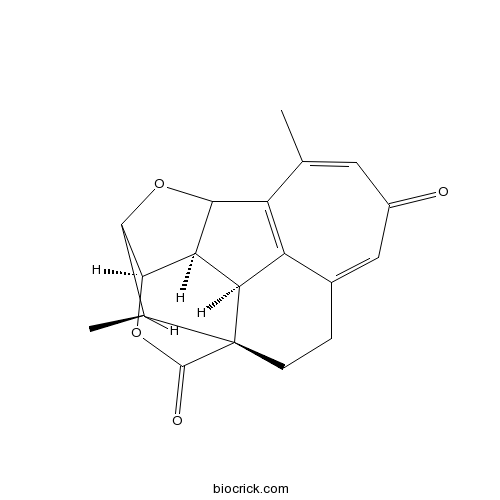
Chemical structure

3D structure
| Cas No. | 64761-48-4 | SDF | Download SDF |
| PubChem ID | 56841078 | Appearance | Powder |
| Formula | C19H18O4 | M.Wt | 310.3 |
| Type of Compound | Diterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (1S,11S,12R,16R,19R)-8,19-dimethyl-14,17-dioxahexacyclo[13.3.1.01,11.04,10.09,13.012,16]nonadeca-4,7,9-triene-6,18-dione | ||
| SMILES | CC1C2C3C4C5C1(CCC6=CC(=O)C=C(C(=C56)C4O2)C)C(=O)O3 | ||
| Standard InChIKey | QNJIIOHVULPMRL-MBKAUVROSA-N | ||
| Standard InChI | InChI=1S/C19H18O4/c1-7-5-10(20)6-9-3-4-19-8(2)15-17(23-18(19)21)13-14(19)12(9)11(7)16(13)22-15/h5-6,8,13-17H,3-4H2,1-2H3/t8-,13+,14+,15?,16?,17+,19+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Harringtonolide Dilution Calculator

Harringtonolide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.2227 mL | 16.1134 mL | 32.2269 mL | 64.4538 mL | 80.5672 mL |
| 5 mM | 0.6445 mL | 3.2227 mL | 6.4454 mL | 12.8908 mL | 16.1134 mL |
| 10 mM | 0.3223 mL | 1.6113 mL | 3.2227 mL | 6.4454 mL | 8.0567 mL |
| 50 mM | 0.0645 mL | 0.3223 mL | 0.6445 mL | 1.2891 mL | 1.6113 mL |
| 100 mM | 0.0322 mL | 0.1611 mL | 0.3223 mL | 0.6445 mL | 0.8057 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Taraxinic acid
Catalog No.:BCN9573
CAS No.:75911-33-0
- 1,7-Bis(4-hydroxyphenyl)hepta-1,4,6-trien-3-one
Catalog No.:BCN9572
CAS No.:149732-52-5
- Isohopeaphenol
Catalog No.:BCN9571
CAS No.:197446-77-8
- Hopeaphenol
Catalog No.:BCN9570
CAS No.:388582-37-4
- Myricetin 3,7,3'-trimethyl ether 5'-O-glucoside
Catalog No.:BCN9569
CAS No.:2170444-56-9
- 3,4-Dihydroxyallylbenzene 3,4-di-O-glucoside
Catalog No.:BCN9568
CAS No.:454473-97-3
- 3,4,5-Trihydroxyallylbenzene 3,4-di-O-glucoside
Catalog No.:BCN9567
CAS No.:2172431-63-7
- Pukateine
Catalog No.:BCN9566
CAS No.:81-67-4
- 11-Methylforsythide
Catalog No.:BCN9565
CAS No.:159598-00-2
- Adoxoside
Catalog No.:BCN9564
CAS No.:42830-26-2
- 3-O-Methylellagic acid 4-O-rhamnoside
Catalog No.:BCN9563
CAS No.:639089-97-7
- Quercetin 3,5,3'-trimethyl ether
Catalog No.:BCN9562
CAS No.:13459-09-1
- Caryatin
Catalog No.:BCN9575
CAS No.:1486-66-4
- Demethylagrimonolide 6-O-glucoside
Catalog No.:BCN9576
CAS No.:1257408-55-1
- Momordicin IV
Catalog No.:BCN9577
CAS No.:894412-35-2
- Taikuguasin D
Catalog No.:BCN9578
CAS No.:1627163-80-7
- Karaviloside XI
Catalog No.:BCN9579
CAS No.:934739-35-2
- Hainanolidol
Catalog No.:BCN9580
CAS No.:73213-63-5
- Fortunolide A
Catalog No.:BCN9581
CAS No.:252574-51-9
- Actinodaphnine
Catalog No.:BCN9582
CAS No.:517-69-1
- Goyaglycoside d
Catalog No.:BCN9583
CAS No.:333332-50-6
- 3'-Hydroxy-3,5,6,7,8,4',5'-heptamethoxyflavone
Catalog No.:BCN9584
CAS No.:5244-28-0
- (+)-Isoampelopsin F
Catalog No.:BCN9585
CAS No.:354553-38-1
- Runanine
Catalog No.:BCN9586
CAS No.:100485-12-9
Total Synthesis of the Diterpenoid (+)-Harringtonolide.[Pubmed:27529411]
Angew Chem Int Ed Engl. 2016 Sep 12;55(38):11638-41.
Described herein is the first asymmetric total synthesis of (+)-Harringtonolide, a natural diterpenoid with an unusual tropone imbedded in a cagelike framework. The key transformations include an intramolecular Diels-Alder reaction and a rhodium-complex-catalyzed intramolecular [3+2] cycloaddition to install the tetracyclic core as well as a highly efficient tropone formation.
Synthesis of Tetracyclic Diterpenoids with Pharmacologic Relevance.[Pubmed:26728618]
Curr Pharm Des. 2016;22(12):1767-807.
This review covers the chemical reactions, synthesis, and biological activities of tetracyclic diterpenoids including ent-kauranes, ent-beyeranes, ent-atisanes, ingenanes, tyglianes, stemodanes, stemaranes, sordarin, salvileucalin B, Harringtonolide and hainanolidol. It comprises of the un-reviewed references from the year 2000.
Stereoselective total synthesis of hainanolidol and harringtonolide via oxidopyrylium-based [5 + 2] cycloaddition.[Pubmed:23930656]
J Am Chem Soc. 2013 Aug 21;135(33):12434-8.
The tetracyclic carbon skeleton of hainanolidol and Harringtonolide was efficiently constructed by an intramolecular oxidopyrylium-based [5 + 2] cycloaddition. An anionic ring-opening strategy was developed for the cleavage of the ether bridge in 8-oxabicyclo[3.2.1]octenes derived from the [5 + 2] cycloaddition. Conversion of cycloheptadiene to tropone was realized by a sequential [4 + 2] cycloaddition, Kornblum-DeLaMare rearrangement, and double elimination. The biomimetic synthesis of Harringtonolide from hainanolidol was also confirmed.
Asymmetric synthesis of the oxygenated polycyclic system of (+)-harringtonolide.[Pubmed:22339261]
Org Lett. 2012 Mar 2;14(5):1270-3.
A straightforward asymmetric synthesis of the cage oxygenated structure of (+)-Harringtonolide has been accomplished for the first time. The key steps involved (i) a templated stereoselective IMDA reaction to build a highly functionalized cyclohexene ring D, (ii) functionalization of the cycloadduct, (iii) ring-closing metathesis providing the five-membered ring C, and finally (iv) a challenging one-step cascade cyclization of an epoxy-alcohol toward the target structure, whose mechanism was investigated.
Synthesis of novel molecular probes inspired by harringtonolide.[Pubmed:21537512]
Org Biomol Chem. 2011 Jun 21;9(12):4570-9.
A novel Harringtonolide-inspired scaffold containing a cycloheptatriene ring and two fused cyclopentane rings has been synthesised from simple starting materials. The scaffold, containing a similar substitution pattern and relative stereochemistry to the complex diterpenoid, has been enumerated into a small library of derivatives. One of these library members has been converted into a sub-library of substituted triazoles using copper-catalysed azide-alkyne cycloaddition (click) chemistry. The scaffold may be useful in drug discovery or in the preparation of additional molecular probes for chemical biology.
Further studies of the norditerpene (+)-harringtonolide isolated from Cephalotaxus harringtonia var. drupacea: absolute configuration, cytotoxic and antifungal activities.[Pubmed:18523925]
Planta Med. 2008 Jun;74(8):870-2.
Harringtonolide (= hainanolide) is a complex polycyclic fused norditerpene isolated from CEPHALOTAXUS HARRINGTONIA var. DRUPACEA. In spite of its appealing biological properties - we measured an IC (50) of 43 nM on KB cells and a significant antifungal activity - its absolute configuration has not yet been firmly established. This was done herein using X-ray anomalous scattering after bromination of the tropone ring, unambiguously giving the stereochemistry 5 R,6 R,7 S,13 S,14 S,15 R,16 R. Detailed IN VITRO biological measurements are provided.
Model studies toward the synthesis of the bioactive diterpenoid, harringtonolide.[Pubmed:18019538]
Org Biomol Chem. 2007 Aug 21;5(16):2627-35.
In model studies towards the synthesis of Harringtonolide, the construction of the tropone moiety via arene cyclopropanation was investigated. The installation of the lactone ring was accomplished by way of a Diels-Alder cycloaddition of various indenones and a-pyones. The incorporation of the key bridge methyl group and subsequent control of its stereochemistry is also outlined.


