Gypenoside XLVICAS# 94705-70-1 |
Quality Control & MSDS
Number of papers citing our products

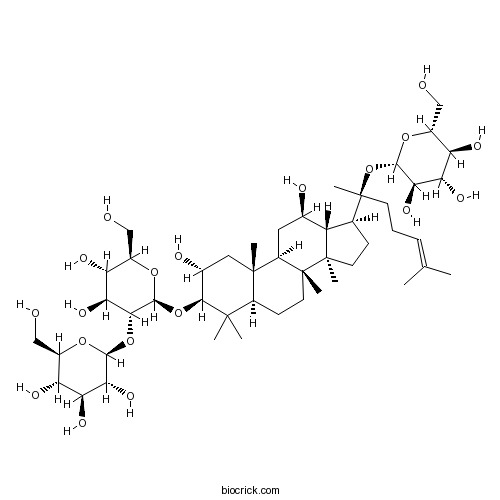
Chemical structure

3D structure
| Cas No. | 94705-70-1 | SDF | Download SDF |
| PubChem ID | 76320987 | Appearance | Powder |
| Formula | C48H82O19 | M.Wt | 963.2 |
| Type of Compound | Triterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2S,3R,4S,5S,6R)-2-[(2R,3R,4S,5S,6R)-2-[[(2R,3R,5R,8R,9R,10R,12R,13R,14R,17S)-2,12-dihydroxy-4,4,8,10,14-pentamethyl-17-[(2S)-6-methyl-2-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyhept-5-en-2-yl]-2,3,5,6,7,9,11,12,13,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3-yl]oxy]-4,5-dihydroxy-6-(hydroxymethyl)oxan-3-yl]oxy-6-(hydroxymethyl)oxane-3,4,5-triol | ||
| SMILES | CC(=CCCC(C)(C1CCC2(C1C(CC3C2(CCC4C3(CC(C(C4(C)C)OC5C(C(C(C(O5)CO)O)O)OC6C(C(C(C(O6)CO)O)O)O)O)C)C)O)C)OC7C(C(C(C(O7)CO)O)O)O)C | ||
| Standard InChIKey | VENRSYBHHVDBDC-YETCIKTFSA-N | ||
| Standard InChI | InChI=1S/C48H82O19/c1-21(2)10-9-13-48(8,67-42-38(61)35(58)32(55)26(19-50)63-42)22-11-14-47(7)30(22)23(52)16-29-45(5)17-24(53)40(44(3,4)28(45)12-15-46(29,47)6)66-43-39(36(59)33(56)27(20-51)64-43)65-41-37(60)34(57)31(54)25(18-49)62-41/h10,22-43,49-61H,9,11-20H2,1-8H3/t22-,23+,24+,25+,26+,27+,28-,29+,30-,31+,32+,33+,34-,35-,36-,37+,38+,39+,40-,41-,42-,43-,45-,46+,47+,48-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Gypenoside XLVI shows strong cytotoxic activity against A549 cells, with the IC50 values of 52.63±8.31 ug/ml. |

Gypenoside XLVI Dilution Calculator

Gypenoside XLVI Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.0382 mL | 5.191 mL | 10.3821 mL | 20.7641 mL | 25.9551 mL |
| 5 mM | 0.2076 mL | 1.0382 mL | 2.0764 mL | 4.1528 mL | 5.191 mL |
| 10 mM | 0.1038 mL | 0.5191 mL | 1.0382 mL | 2.0764 mL | 2.5955 mL |
| 50 mM | 0.0208 mL | 0.1038 mL | 0.2076 mL | 0.4153 mL | 0.5191 mL |
| 100 mM | 0.0104 mL | 0.0519 mL | 0.1038 mL | 0.2076 mL | 0.2596 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Rhodiolgin; Gossypetin-7-O-α-rhamnopyranoside
Catalog No.:BCC8247
CAS No.:94696-39-6
- MNI 137
Catalog No.:BCC6156
CAS No.:946619-21-2
- Hyperectine
Catalog No.:BCN3406
CAS No.:94656-46-9
- LY2409881
Catalog No.:BCC5650
CAS No.:946518-60-1
- RN 1734
Catalog No.:BCC7770
CAS No.:946387-07-1
- Mulberrofuran K
Catalog No.:BCN7188
CAS No.:94617-36-4
- RO5126766(CH5126766)
Catalog No.:BCC6374
CAS No.:946128-88-7
- LX-1031
Catalog No.:BCC1712
CAS No.:945976-76-1
- Senkyunolide I
Catalog No.:BCN6353
CAS No.:94596-28-8
- Senkyunolide H
Catalog No.:BCN6352
CAS No.:94596-27-7
- Parisyunnanoside B
Catalog No.:BCN2837
CAS No.:945865-37-2
- AA 29504
Catalog No.:BCC7829
CAS No.:945828-50-2
- PF 429242
Catalog No.:BCC6009
CAS No.:947303-87-9
- Fmoc-Aib-OH
Catalog No.:BCC3149
CAS No.:94744-50-0
- Salmeterol xinafoate
Catalog No.:BCC1920
CAS No.:94749-08-3
- 7,3'-Dihydroxy-5'-methoxyisoflavone
Catalog No.:BCN3349
CAS No.:947611-61-2
- WWL 70
Catalog No.:BCC4011
CAS No.:947669-91-2
- TCFH
Catalog No.:BCC2824
CAS No.:94790-35-9
- HBTU
Catalog No.:BCC2814
CAS No.:94790-37-1
- ML365
Catalog No.:BCC8063
CAS No.:947914-18-3
- 3-pyr-Cytisine
Catalog No.:BCC6118
CAS No.:948027-43-8
- Bruceantinol A
Catalog No.:BCN8003
CAS No.:948038-36-6
- Bruceine J
Catalog No.:BCN8001
CAS No.:948038-38-8
- 20(R)-Notoginsenoside R2
Catalog No.:BCN3864
CAS No.:948046-15-9
Comparison of the Effects and Inhibitory Pathways of the Constituents from Gynostemma pentaphyllum against LPS-Induced Inflammatory Response.[Pubmed:30301351]
J Agric Food Chem. 2018 Oct 31;66(43):11337-11346.
Saponins, the primary phytochemicals contributing to the health properties of G. pentaphyllum were frequently studied. However, compounds responsible for its bioactivities were still poorly understood. The saponin-rich fraction (GPMS), 3- O-[2G-( E)-Coumaroyl-3G- O-beta-d-glucosyl-3R- O-beta-d-glucosylrutinoside] (KCGG), Gypenoside XLVI and gypenoside L were obtained by purification of G. pentaphyllum. The compounds were examined and compared with GPMS for their inhibitory effects on LPS-induced nitric oxide (NO) production. GPMS and KCGG differed in their inhibitory capacities against pro-inflammatory cytokines secretion. GPMS exhibited strong inhibition on inducible nitric oxide synthase (iNOS) and interleukin-6 (IL-6) mRNA expression but weak inhibition on tumor necrosis factor-alpha (TNF-alpha) and interleukin-1beta mRNA expression. KCGG was better at inhibiting iNOS, IL-6, TNF-alpha, and cyclooxygenase-2 (COX-2) mRNA expression. GPMS showed similar inhibitory potency on mitogen-activated protein kinase phosphorylation and nuclear factor-kappaB (NF-kappaB) activation, as evidenced by their regulatory effects on LPS-induced P65 phosphorylation, NF-kappaB nuclear translocation, IkappaBalpha phosphorylation and degradation, IkappaKalpha/beta phosphorylation, c-Jun N-terminal kinase phosphorylation, P38 phosphorylation, and COX-2 expression. KCGG was more powerful in inhibiting the NF-kappaB pathway, suggesting that KCGG might be used in the management of inflammatory-associated diseases in which NF-kappaB played pivotal roles. Furthermore, KCGG might be mainly responsible for the predominant effects of GPMS.
Gynosaponin TN-1 producing from the enzymatic conversion of gypenoside XLVI by naringinase and its cytotoxicity on hepatoma cell lines.[Pubmed:29751078]
Food Chem Toxicol. 2018 Sep;119:161-168.
Gypenoside XLVI (gyp XLVI) is one of the major dammarane-type triterpenoid saponins from Gynostamma pentaphallum with glucosyls at C-3 and C-20 positions, which may constrain its bioactivities. The enzymatic conversion of gyp XLVI by naringinase, and the cytotoxicity of enzymolysis product on SMMC7721 and Bel7402 hepatoma cells were investigated. The results showed that gynosaponin TN-1 (gyp TN-1) was produced from the enzymatic conversion of gyp XLVI by naringinase. The optimum enzymolysis conditions were pH 4.2, 47.3 degrees C, and 16h, with a yield of 73.44+/-6.52% for gyp TN-1. In addition, gyp TN-1 exhibited higher inhibitory activities on SMMC7721 and Bel7402 hepatoma cells than gyp XLVI. Results from methyl thiazolyl tetrazolium (MTT) assay and acridine orange (AO)/ethidium bromide (EB) double staining were highly consistent. These results demonstrated that enzymatic conversion could be a promising method for producing gyp TN-1 from the biotransformation of gyp XLVI and the preparation of gyp TN-1 might provide a reference for the acquisition of novel anticancer drugs.
Simultaneous determination of gypenoside LVI, gypenoside XLVI, 2alpha-OH-protopanaxadiol and their two metabolites in rat plasma by LC-MS/MS and its application to pharmacokinetic studies.[Pubmed:26454343]
J Chromatogr B Analyt Technol Biomed Life Sci. 2015 Nov 15;1005:9-16.
Gypenoside LVI and Gypenoside XLVI are the major bioactive dammarane saponins from Gynostemma pentaphyllum. Gypenoside LVI, Gypenoside XLVI, and their metabolite 2alpha-OH-protopanaxadiol (2alpha-OH-PPD) possess potent non-small cell lung carcinoma A549 cell inhibitory activity. A sensitive liquid chromatography tandem mass spectrometry method was developed and validated to study the pharmacokinetics of gypenoside LVI and XLVI, 2alpha-OH-PPD, metabolite 1 (M1), and metabolite 2 (M2) after administration of gypenosides or 2alpha-OH-PPD. Plasma samples from rats were protein precipitated with methanol. Analytes were detected by triple quadrupole MS/MS with an electrospray ionization source in the positive multiple reaction monitoring mode. The transition m/z 441.4-->109.2 was selected to quantify gypenoside LVI and XLVI, and 2alpha-OH-PPD, because of the extensive conversion of the gypenosides to aglycone in the ionization source. M1 and M2 are isomers that shared the transition m/z 493.4-->143.1. To avoid interference, the baseline separation of each analyte was performed on a SunFire C18 column with a gradient of acetonitrile (0.1% formic acid, v/v) and water (0.1% formic acid, v/v). The chromatographic run time was 10min. The linearity was validated over a plasma concentration range from 2.00 to 2000ng/mL for M1 and M2, and from 10.0 to 2000 for gypenosides LVI and XLVI, and 2alpha-OH-protopanaxadiol. The lower limits of quantification were 10.0, 10.0, 10.0, 2.00, and 2.00ng/mL for gypenoside LVI, Gypenoside XLVI, 2alpha-OH-PPD, M1, and M2, respectively, with acceptable intra-/inter-day precision and accuracy. The extraction recovery rates were >86.9% for each compound. No apparent matrix effect or instability was observed during each step of the bioanalysis. After full validation, this method was proved to be simple, fast, and efficient in analyzing large batches of plasma samples for the analytes.
Determination by UPLC-MS of four dammarane-type saponins from heat-processed Gynostemma pentaphyllum.[Pubmed:25036687]
Biosci Biotechnol Biochem. 2014;78(2):311-6.
Heat-processed Gynostemma pentaphyllum and its main dammaran-type saponins, gypenoside L, gypenoside LI, damulin B, and damulin A, possess non-small cell lung carcinoma A549 cell inhibitory activity. We established in this study a method by ultra-high performance liquid chromatography with tandem mass spectrometry for determination of the saponins and also investigated their content change in heat-processed G. pentaphyllum. The main saponins increased with increasing heating temperature and time. Further investigation showed that they were produced from Gypenoside XLVI and gypenoside LVI by undergoing hydrolysis during the heat treatment.
Dammarane-type glycosides and long chain sesquiterpene glycosides from Gynostemma yixingense.[Pubmed:19781603]
Fitoterapia. 2010 Jun;81(4):248-52.
A new dammarane-type glycoside and a new long chain sesquiterpene glycoside, along with nine known compounds 20(S)-ginsenoside Rh1 (3), 20(R)-ginsenoside Rh1 (4), ginsenoside F1 (4), amarantholidoside IV (6), ginsenoside Rc (7), 20(S)-ginsenoside Rg2 (8), 20(R)-ginsenoside Rg2 (9), ginsenoside Rd (10) and Gypenoside XLVI (11) were isolated from Gynostemma yixingense. The structures of the new compounds were determined on the basis of spectroscopic analysis, including 1D-, 2D-NMR and ESI-MS techniques as well as by comparison of the spectral data with those of related compounds as 2 alpha,3beta,20(S)-trihydroxydammar-24-ene-3-O-[beta-D-glucopyranosyl((1-->2)-beta -D-glucopyranosyl]-20-O-[beta-D-xylopyranosyl((1-->6)-beta-D-glucopyranoside] (1) (2E,6E)-10-beta-D-glucopyranosyl-1,10,11-trihydroxy-3,7,11-trimethyldodeca-2,6-di ene (2).


