Ginsenoside Rd2CAS# 83480-64-2 |
- Stevenleaf
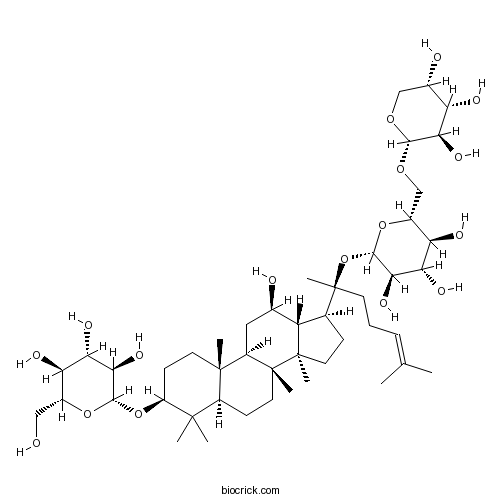
Catalog No.:BCN5978
CAS No.:80321-63-7
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 83480-64-2 | SDF | Download SDF |
| PubChem ID | 21672569 | Appearance | White powder |
| Formula | C47H80O17 | M.Wt | 917.13 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Quinquenoside L10 | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2R,3S,4S,5R,6R)-2-(hydroxymethyl)-6-[[(3S,5R,8R,9R,10R,12R,13R,14R,17S)-12-hydroxy-4,4,8,10,14-pentamethyl-17-[(2S)-6-methyl-2-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-[[(2S,3R,4S,5S)-3,4,5-trihydroxyoxan-2-yl]oxymethyl]oxan-2-yl]oxyhept-5-en-2-yl]-2,3,5,6,7,9,11,12,13,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3-yl]oxy]oxane-3,4,5-triol | ||
| SMILES | CC(=CCCC(C)(C1CCC2(C1C(CC3C2(CCC4C3(CCC(C4(C)C)OC5C(C(C(C(O5)CO)O)O)O)C)C)O)C)OC6C(C(C(C(O6)COC7C(C(C(CO7)O)O)O)O)O)O)C | ||
| Standard InChIKey | ZTQSADJAYQOCDD-FDDSVCGKSA-N | ||
| Standard InChI | InChI=1S/C47H80O17/c1-22(2)10-9-14-47(8,64-42-39(58)36(55)34(53)27(62-42)21-60-40-37(56)32(51)25(50)20-59-40)23-11-16-46(7)31(23)24(49)18-29-44(5)15-13-30(43(3,4)28(44)12-17-45(29,46)6)63-41-38(57)35(54)33(52)26(19-48)61-41/h10,23-42,48-58H,9,11-21H2,1-8H3/t23-,24+,25-,26+,27+,28-,29+,30-,31-,32-,33+,34+,35-,36-,37+,38+,39+,40-,41-,42-,44-,45+,46+,47-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Ginsenoside Rd2 Dilution Calculator

Ginsenoside Rd2 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.0904 mL | 5.4518 mL | 10.9036 mL | 21.8072 mL | 27.2589 mL |
| 5 mM | 0.2181 mL | 1.0904 mL | 2.1807 mL | 4.3614 mL | 5.4518 mL |
| 10 mM | 0.109 mL | 0.5452 mL | 1.0904 mL | 2.1807 mL | 2.7259 mL |
| 50 mM | 0.0218 mL | 0.109 mL | 0.2181 mL | 0.4361 mL | 0.5452 mL |
| 100 mM | 0.0109 mL | 0.0545 mL | 0.109 mL | 0.2181 mL | 0.2726 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Voglibose
Catalog No.:BCC4750
CAS No.:83480-29-9
- Mifamurtide
Catalog No.:BCC5241
CAS No.:83461-56-7
- Ginsenoside Ra1
Catalog No.:BCN8392
CAS No.:83459-41-0
- Ebracteolata cpd B
Catalog No.:BCN3781
CAS No.:83459-37-4
- Boc-D-Prolinol
Catalog No.:BCC2707
CAS No.:83435-58-9
- ICI 154,129
Catalog No.:BCC5677
CAS No.:83420-94-4
- Phenformin HCl
Catalog No.:BCC4362
CAS No.:834-28-6
- PL 017
Catalog No.:BCC5864
CAS No.:83397-56-2
- Prenyl caffeate
Catalog No.:BCC8351
CAS No.:100884-13-7
- D609
Catalog No.:BCC1509
CAS No.:83373-60-8
- 25(R)-Hydroxyprotopanaxadiol
Catalog No.:BCN2493
CAS No.:83349-37-5
- 10-O-Caffeoyl-6-epiferetoside
Catalog No.:BCN4822
CAS No.:83348-22-5
- Grantaline
Catalog No.:BCN2083
CAS No.:83482-61-5
- 4-[4-(3-Hydroxyphenyl)-3-(4-methylphenyl)-6-oxo-1,4-dihydropyrrolo[3,4-d]pyrazol-5-yl]benzoic acid
Catalog No.:BCC6341
CAS No.:834903-43-4
- Leucosceptoside A
Catalog No.:BCN7457
CAS No.:83529-62-8
- Anisofolin A
Catalog No.:BCN4377
CAS No.:83529-71-9
- 2,2-Bis(3-amino-4-hydroxyphenyl)hexafluoropropane
Catalog No.:BCC8490
CAS No.:83558-87-6
- Regorafenib hydrochloride
Catalog No.:BCC1883
CAS No.:835621-07-3
- Cropodine
Catalog No.:BCN2073
CAS No.:83601-85-8
- Cyclosporin H
Catalog No.:BCC6448
CAS No.:83602-39-5
- (S)-AMPA
Catalog No.:BCC6583
CAS No.:83643-88-3
- CGRP (rat)
Catalog No.:BCC5712
CAS No.:83651-90-5
- RHC 80267
Catalog No.:BCC8083
CAS No.:83654-05-1
- (R)-AMPA
Catalog No.:BCC6582
CAS No.:83654-13-1
Comparative study on chemical components and anti-inflammatory effects of Panax notoginseng flower extracted by water and methanol.[Pubmed:29068139]
J Sep Sci. 2017 Dec;40(24):4730-4739.
Methanol and water are commonly used solvents for chemical analysis and traditional decoction, respectively. In the present study, a high-performance liquid chromatography with ultraviolet detection method was developed to quantify 11 saponins in Panax notoginseng flower extracted by aqueous solution and methanol, and chemical components and anti-inflammatory effects of these two extracts were compared. The separation of 11 saponins, including notoginsenoside Fc and ginsenoside Rc, was well achieved on a Zorbax SB C18 column. This developed method provides an adequate linearity (r(2) > 0.999), repeatability (RSD < 4.26%), inter- and intraday variations (RSD < 3.20%) with recovery (94.7-104.1%) of 11 saponins concerned. Our data indicated that ginsenoside biotransformation in PNF was found, when water was used as the extraction solvent, but not methanol. Specifically, the major components of Panax notoginseng flower, ginsenosides Rb1, Rc, Rb2, Rb3, and Rd, can be near completely transformed to the minor components, gypenoside XVII, notoginsenoside Fe, Ginsenoside Rd2, notoginsenoside Fd, and ginsenoside F2, respectively. Total protein isolated from Panax notoginseng flower is responsible for this ginsenoside biotransformation. Additionally, methanol extract exerted the stronger anti-inflammatory effects than water extract in lipopolysaccharide-induced RAW264.7 cells. This difference in anti-inflammatory action might be attributed to their chemical difference of saponins.
[Chemical constituents of leaves of Panax japonicus var. major].[Pubmed:25095375]
Zhongguo Zhong Yao Za Zhi. 2014 May;39(9):1635-8.
Seven compounds were isolated from the leaves of Panax japonicus var. major by chromatographic methods including silica gel, Sephadex LH-20, ODS and semi-preparative HPLC. Their structures were elucidated by their physical and chemical properties and spectral data analysis as 5, 7-dihydroxy-8-methoxyl flavone (1), ginsenoside Rs2 (2), quinquenoside R1 (3), ginsenoside Rs1 (4), notoginsenoside Fe (5), Ginsenoside Rd2 (6) and gypenosiden IX (7). Among them, compound 1 was obtained from the Panax genus for the first time, and compounds 2-7 were isolated from this plant for the first time.


