GW788388ALK5 inhibitor,potent and selective CAS# 452342-67-5 |
Quality Control & MSDS
Number of papers citing our products

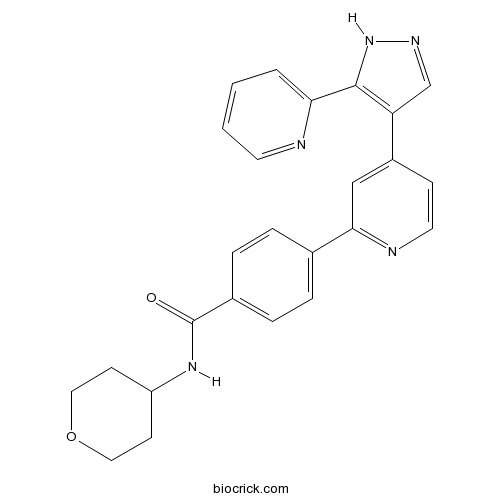
Chemical structure
3D structure
| Cas No. | 452342-67-5 | SDF | Download SDF |
| PubChem ID | 10202642 | Appearance | Powder |
| Formula | C25H23N5O2 | M.Wt | 425.49 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 48 mg/mL (112.81 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | N-(oxan-4-yl)-4-[4-(5-pyridin-2-yl-1H-pyrazol-4-yl)pyridin-2-yl]benzamide | ||
| SMILES | C1COCCC1NC(=O)C2=CC=C(C=C2)C3=NC=CC(=C3)C4=C(NN=C4)C5=CC=CC=N5 | ||
| Standard InChIKey | SAGZIBJAQGBRQA-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C25H23N5O2/c31-25(29-20-9-13-32-14-10-20)18-6-4-17(5-7-18)23-15-19(8-12-27-23)21-16-28-30-24(21)22-3-1-2-11-26-22/h1-8,11-12,15-16,20H,9-10,13-14H2,(H,28,30)(H,29,31) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent inhibitor of transforming growth factor-β type I receptor (ALK5) (IC50 values are 18 and 93 nM for ALK5 binding and for TGF-β cellular assay respectively). Inhibits esophageal squamous cell carcinoma (ESCC)-induced neoangiogenesis. Orally active. |

GW788388 Dilution Calculator

GW788388 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3502 mL | 11.7512 mL | 23.5023 mL | 47.0046 mL | 58.7558 mL |
| 5 mM | 0.47 mL | 2.3502 mL | 4.7005 mL | 9.4009 mL | 11.7512 mL |
| 10 mM | 0.235 mL | 1.1751 mL | 2.3502 mL | 4.7005 mL | 5.8756 mL |
| 50 mM | 0.047 mL | 0.235 mL | 0.47 mL | 0.9401 mL | 1.1751 mL |
| 100 mM | 0.0235 mL | 0.1175 mL | 0.235 mL | 0.47 mL | 0.5876 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
GW788388 is a selective inhibitor of ALK5 with IC50 value of 18 nM [1].
Transforming growth factor beta (TGF-beta) type I receptor (ALK5) is the receptor of TGF-beta and plays an important role in transducing the TGF-beta signal from the cell surface to the cytoplasm [2 ].
GW788388 is a potent TGF-beta type I receptor inhibitor and has a much improved pharmacokinetic profile compared with the reported TGF-beta type I receptor inhibitor SB431542. When tested with human embryonic kidney 293T cells transfected with ALK5, TβRII, BMPRII or ActRII, GW788388 exhibited specific inhibitory function on the autophosphorylation of ALK5 and TβRII, while had some extent to ActRII and had no effect on BMPRII. Further, using Namrumurine mammary gland (NMuMG), MDA-MB-231, renal cell carcinoma (RCC)4 and U2OS cell lines, GW788388 treatment inhibited TGF-β-induced Smad2 phosphorylation, inhibited TGF-β-induced EMT and growth, and TGF-β-induced fibrotic responses [1]. In ESCC/fibroblast/HMVEC co-culture model, GW788388 treatment (1 μM) resulted in a complete reversal of vascular network formation that indicated GW788388 blocked ESCC-induced neoangiogenesis [3].
In 6-month-old db/db mouse model of spontaneous diabetic nephropathy, administration of GW788388 at the dose of 2 mg/kg/day orally for 5 weeks attenuated renal fibrosis without any side-effect [1]. In 10-week Sprague-Dawley rats model, oral administration of GW788388 (100-1000 mg/kg/day) for 4 days induced the thickness of femoral physis in a dose-dependent manner and severity of physeal changes , as well as subphyseal hyperostosis, increased with duration of dosing progressing from minimal to moderate in rats given 300 mg/kg/day for 10 days [2].
References:
[1]. Petersen, M., et al., Oral administration of GW788388, an inhibitor of TGF-beta type I and II receptor kinases, decreases renal fibrosis. Kidney Int, 2008. 73(6): p. 705-15.
[2]. Frazier, K., et al., Inhibition of ALK5 signaling induces physeal dysplasia in rats. Toxicol Pathol, 2007. 35(2): p. 284-95.
[3]. Noma, K., et al., The essential role of fibroblasts in esophageal squamous cell carcinoma-induced angiogenesis. Gastroenterology, 2008. 134(7): p. 1981-93.
- SU14813 double bond Z
Catalog No.:BCC1972
CAS No.:452105-23-6
- Boc-N-Me-Val-OH
Catalog No.:BCC3357
CAS No.:45170-31-8
- AV-412
Catalog No.:BCC5119
CAS No.:451493-31-5
- AV-412 free base
Catalog No.:BCC5120
CAS No.:451492-95-8
- 10-Hydroxydihydroperaksine
Catalog No.:BCN5502
CAS No.:451478-47-0
- H-1152
Catalog No.:BCC1615
CAS No.:451462-58-1
- H-D-Glu(OtBu)-OH
Catalog No.:BCC2942
CAS No.:45125-00-6
- H-Glu-OtBu
Catalog No.:BCC2925
CAS No.:45120-30-7
- H-Aib-OtBu.HCl
Catalog No.:BCC3208
CAS No.:4512-32-7
- H-Met-NH2
Catalog No.:BCC2994
CAS No.:4510-08-1
- Homogentisic acid
Catalog No.:BCN3889
CAS No.:451-13-8
- FAK Inhibitor 14
Catalog No.:BCC7683
CAS No.:4506-66-5
- Boc-DL-Phe-OH
Catalog No.:BCC3434
CAS No.:4530-18-1
- Boc-Gly-OH
Catalog No.:BCC3396
CAS No.:4530-20-5
- Saprirearine
Catalog No.:BCN3980
CAS No.:453518-30-4
- Motesanib
Catalog No.:BCC1776
CAS No.:453562-69-1
- PSB 11 hydrochloride
Catalog No.:BCC7239
CAS No.:453591-58-7
- Nudifloside D
Catalog No.:BCN7005
CAS No.:454212-54-5
- kobe2602
Catalog No.:BCC5291
CAS No.:454453-49-7
- Acetophenone tosylhydrazone
Catalog No.:BCC8804
CAS No.:4545-21-5
- Corosolic acid
Catalog No.:BCN5503
CAS No.:4547-24-4
- Grossamide K
Catalog No.:BCC4547
CAS No.:
- 4-Benzoyl-3-methyl-1-phenyl-5-pyrazolone
Catalog No.:BCC8695
CAS No.:4551-69-3
- Isovouacapenol C
Catalog No.:BCN6557
CAS No.:455255-15-9
Oral administration of GW788388, an inhibitor of TGF-beta type I and II receptor kinases, decreases renal fibrosis.[Pubmed:18075500]
Kidney Int. 2008 Mar;73(6):705-15.
Progressive kidney fibrosis precedes end-stage renal failure in up to a third of patients with diabetes mellitus. Elevated intra-renal transforming growth factor-beta (TGF-beta) is thought to underlie disease progression by promoting deposition of extracellular matrix and epithelial-mesenchymal transition. GW788388 is a new TGF-beta type I receptor inhibitor with a much improved pharmacokinetic profile compared with SB431542. We studied its effect in vitro and found that it inhibited both the TGF-beta type I and type II receptor kinase activities, but not that of the related bone morphogenic protein type II receptor. Further, it blocked TGF-beta-induced Smad activation and target gene expression, while decreasing epithelial-mesenchymal transitions and fibrogenesis. Using db/db mice, which develop diabetic nephropathy, we found that GW788388 given orally for 5 weeks significantly reduced renal fibrosis and decreased the mRNA levels of key mediators of extracellular matrix deposition in kidneys. Our study shows that GW788388 is a potent and selective inhibitor of TGF-beta signalling in vitro and renal fibrosis in vivo.
Oral administration of GW788388, an inhibitor of transforming growth factor beta signaling, prevents heart fibrosis in Chagas disease.[Pubmed:22720109]
PLoS Negl Trop Dis. 2012;6(6):e1696.
BACKGROUND: Chagas disease induced by Trypanosoma cruzi (T. cruzi) infection is a major cause of mortality and morbidity affecting the cardiovascular system for which presently available therapies are largely inadequate. Transforming Growth Factor beta (TGFss) has been involved in several regulatory steps of T. cruzi invasion and in host tissue fibrosis. GW788388 is a new TGFss type I and type II receptor kinase inhibitor that can be orally administered. In the present work, we studied its effects in vivo during the acute phase of experimental Chagas disease. METHODOLOGY/PRINCIPAL FINDINGS: Male Swiss mice were infected intraperitoneally with 10(4) trypomastigotes of T. cruzi (Y strain) and evaluated clinically. We found that this compound given once 3 days post infection (dpi) significantly decreased parasitemia, increased survival, improved cardiac electrical conduction as measured by PR interval in electrocardiography, and restored connexin43 expression. We could further show that cardiac fibrosis development, evaluated by collagen type I and fibronectin expression, could be inhibited by this compound. Interestingly, we further demonstrated that administration of GW788388 at the end of the acute phase (20 dpi) still significantly increased survival and decreased cardiac fibrosis (evaluated by Masson's trichrome staining and collagen type I expression), in a stage when parasite growth is no more central to this event. CONCLUSION/SIGNIFICANCE: This work confirms that inhibition of TGFss signaling pathway can be considered as a potential alternative strategy for the treatment of the symptomatic cardiomyopathy found in the acute and chronic phases of Chagas disease.
Discovery of 4-{4-[3-(pyridin-2-yl)-1H-pyrazol-4-yl]pyridin-2-yl}-N-(tetrahydro-2H- pyran-4-yl)benzamide (GW788388): a potent, selective, and orally active transforming growth factor-beta type I receptor inhibitor.[Pubmed:16570917]
J Med Chem. 2006 Apr 6;49(7):2210-21.
Inhibitors of transforming growth factor beta (TGF-beta) type I receptor (ALK5) offer a novel approach for the treatment of fibrotic diseases such as renal, hepatic, and pulmonary fibrosis. The optimization of a novel phenylpyridine pyrazole series (1a) led to the identification of potent, selective, and orally active ALK5 inhibitors. The cellular potency and pharmacokinetics profiles of these derivatives were improved and several compounds presented antifibrotic activity when orally administered to rats in an acute liver model of dimethylnitrosamine- (DMN-) induced expression of collagen IA1 mRNA, a major gene contributing to excessive extra cellular matrix deposit. One of the most potent ALK5 inhibitors identified in this chemical series, compound 13d (GW788388), reduced the expression of collagen IA1 by 80% at a dose of 1 mg/kg twice a day (b.i.d.). This compound significantly reduced the expression of collagen IA1 mRNA when administered orally at 10 mg/kg once a day (u.i.d.) in a model of puromycin aminonucleoside-induced renal fibrosis.
Targeted inhibition of activin receptor-like kinase 5 signaling attenuates cardiac dysfunction following myocardial infarction.[Pubmed:20154262]
Am J Physiol Heart Circ Physiol. 2010 May;298(5):H1415-25.
Following myocardial infarction (MI), the heart undergoes a pathological process known as remodeling, which in many instances results in cardiac dysfunction and ultimately heart failure and death. Transforming growth factor-beta (TGF-beta) is a key mediator in the pathogenesis of cardiac remodeling following MI. We thus aimed to inhibit TGF-beta signaling using a novel orally active TGF-beta type I receptor [activin receptor-like kinase 5 (ALK5)] inhibitor (GW788388) to attenuate left ventricular remodeling and cardiac dysfunction in a rat model of MI. Sprague-Dawley rats underwent left anterior descending coronary artery ligation to induce experimental MI and then were randomized to receive GW788388 at a dosage of 50 mg.kg(-1).day(-1) or vehicle 1 wk after surgery. After 4 wk of treatment, echocardiography was performed before the rats were euthanized. Animals that received left anterior descending coronary artery ligation demonstrated systolic dysfunction, Smad2 activation, myofibroblasts accumulation, collagen deposition, and myocyte hypertrophy (all P < 0.05). Treatment with GW788388 significantly attenuated systolic dysfunction in the MI animals, together with the attenuation of the activated (phosphorylated) Smad2 (P < 0.01), alpha-smooth muscle actin (P < 0.001), and collagen I (P < 0.05) in the noninfarct zone of MI rats. Cardiomyocyte hypertrophy in MI hearts was also attenuated by ALK5 inhibition (P < 0.05). In brief, treatment with a novel TGF-beta type I receptor inhibitor, GW788388, significantly reduced TGF-beta activity, leading to the attenuation of systolic dysfunction and left ventricular remodeling in an experimental rat model of MI.
The essential role of fibroblasts in esophageal squamous cell carcinoma-induced angiogenesis.[Pubmed:18439605]
Gastroenterology. 2008 Jun;134(7):1981-93.
BACKGROUND & AIMS: Esophageal squamous cell carcinoma (ESCC) is known to be a highly angiogenic tumor. Here, we investigated the role of the stromal fibroblasts in the ESCC-induced angiogenic response using a novel 3-dimensional model. METHODS: A novel assay was developed where cocultures of ESCC and esophageal fibroblasts induced human microvascular endothelial cell (HMVEC) vascular network formation in a 3-dimensional collagen gel. Biochemical studies showed that the ESCC-induced activation of the fibroblasts was required to induce vascular network formation via a transforming growth factor (TGF)-beta and vascular endothelial growth factor (VEGF)-dependent pathway. RESULTS: Conditioned media from a panel of 4 ESCC lines transdifferentiated normal esophageal fibroblasts into myofibroblasts via TGF-beta signaling. The presence of fibroblasts was essential for efficient HMVEC network formation, and the addition of ESCC cells to these cultures greatly enhanced the angiogenic process. The role of TGF-beta in this process was shown by the complete inhibition of network formation following TGF-beta inhibitor treatment. Finally, we showed that ESCC-derived TGF-beta regulates angiogenesis through the release of VEGF from the fibroblasts and that the VEGF release was blocked following TGF-beta inhibition. CONCLUSIONS: This study shows the essential role of fibroblasts in the ESCC angiogenic-induced response and suggests that the pharmacologic targeting of the TGF-beta signaling axis could be of therapeutic benefit in this deadly disease.


