Fmoc-Thr(Trt)-OHCAS# 133180-01-5 |
- PF-4708671
Catalog No.:BCC5031
CAS No.:1255517-76-0
- BIX 02565
Catalog No.:BCC4303
CAS No.:1311367-27-7
- BI-D1870
Catalog No.:BCC5030
CAS No.:501437-28-1
- CMK
Catalog No.:BCC1489
CAS No.:821794-90-5
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
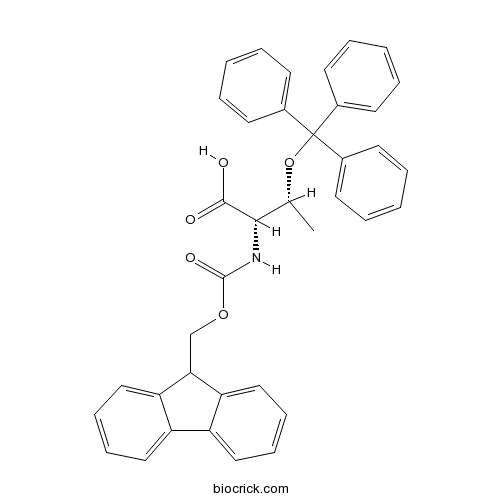
| Cas No. | 133180-01-5 | SDF | Download SDF |
| PubChem ID | 11180767 | Appearance | Powder |
| Formula | C38H33NO5 | M.Wt | 583.7 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2S,3R)-2-(9H-fluoren-9-ylmethoxycarbonylamino)-3-trityloxybutanoic acid | ||
| SMILES | CC(C(C(=O)O)NC(=O)OCC1C2=CC=CC=C2C3=CC=CC=C13)OC(C4=CC=CC=C4)(C5=CC=CC=C5)C6=CC=CC=C6 | ||
| Standard InChIKey | JARBLLDDSTVWSM-IJAHGLKVSA-N | ||
| Standard InChI | InChI=1S/C38H33NO5/c1-26(44-38(27-15-5-2-6-16-27,28-17-7-3-8-18-28)29-19-9-4-10-20-29)35(36(40)41)39-37(42)43-25-34-32-23-13-11-21-30(32)31-22-12-14-24-33(31)34/h2-24,26,34-35H,25H2,1H3,(H,39,42)(H,40,41)/t26-,35+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Fmoc-Thr(Trt)-OH Dilution Calculator

Fmoc-Thr(Trt)-OH Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.7132 mL | 8.566 mL | 17.1321 mL | 34.2642 mL | 42.8302 mL |
| 5 mM | 0.3426 mL | 1.7132 mL | 3.4264 mL | 6.8528 mL | 8.566 mL |
| 10 mM | 0.1713 mL | 0.8566 mL | 1.7132 mL | 3.4264 mL | 4.283 mL |
| 50 mM | 0.0343 mL | 0.1713 mL | 0.3426 mL | 0.6853 mL | 0.8566 mL |
| 100 mM | 0.0171 mL | 0.0857 mL | 0.1713 mL | 0.3426 mL | 0.4283 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Fmoc-Thr(Trt)-OH
- Fmoc-Cit-OH
Catalog No.:BCC3180
CAS No.:133174-15-9
- Oxypeucedan hydrate
Catalog No.:BCN8372
CAS No.:133164-11-1
- GR 73632
Catalog No.:BCC5801
CAS No.:133156-06-6
- Epimedin B1
Catalog No.:BCN8199
CAS No.:133137-58-3
- Flutamide
Catalog No.:BCC4364
CAS No.:13311-84-7
- Darifenacin HBr
Catalog No.:BCC4567
CAS No.:133099-07-7
- Darifenacin
Catalog No.:BCC1516
CAS No.:133099-04-4
- 3PO
Catalog No.:BCC5616
CAS No.:13309-08-5
- Crassifoline methine
Catalog No.:BCN1793
CAS No.:133084-00-1
- Fmoc-D-Lys(Boc)-OPfp
Catalog No.:BCC3527
CAS No.:133083-36-0
- BIM 23052
Catalog No.:BCC5945
CAS No.:133073-82-2
- ZD 7288
Catalog No.:BCC6884
CAS No.:133059-99-1
- PI3k-delta inhibitor 1
Catalog No.:BCC1861
CAS No.:1332075-63-4
- AM 92016 hydrochloride
Catalog No.:BCC6825
CAS No.:133229-11-5
- Heliangin
Catalog No.:BCN6487
CAS No.:13323-48-3
- 4-O-Methylbutein
Catalog No.:BCN6677
CAS No.:13323-67-6
- GSK2194069
Catalog No.:BCC8053
CAS No.:1332331-08-4
- KPT-185
Catalog No.:BCC4444
CAS No.:1333151-73-7
- TRPC6 inhibitor
Catalog No.:BCC4199
CAS No.:1333207-63-8
- 10-Deacetylyunnanxane
Catalog No.:BCN7338
CAS No.:1333323-17-3
- Imbricataflavone A
Catalog No.:BCN8025
CAS No.:133336-96-6
- CHR-6494
Catalog No.:BCC1479
CAS No.:1333377-65-3
- Lactacystin
Catalog No.:BCN1841
CAS No.:133343-34-7
- Lydicamycin
Catalog No.:BCN1843
CAS No.:133352-27-9
Chemical synthesis and receptor binding of catfish somatostatin: a disulfide-bridged beta-D-Galp-(1-->3)-alpha-D-GalpNAc O-glycopeptide.[Pubmed:10667864]
J Pept Res. 2000 Jan;55(1):81-91.
The glycopeptide hormone catfish somatostatin (somatostatin-22) has the amino acid sequence H-Asp-Asn-Thr-Val-Thr-Ser-Lys-Pro-Leu-Asn-Cys-Met-Asn-Tyr-Phe-Trp-Lys-Se r-Arg-Thr-Ala-Cys-OH; it includes a cyclic disulfide connecting the two Cys residues, and the major naturally occurring glycoform contains D-GalNAc and D-Gal O-glycosidically linked to Thr5. The linear sequence was assembled smoothly starting with an Fmoc-Cys(Trt)-PAC-PEG-PS support, using stepwise Fmoc solid-phase chemistry. In addition to the nonglycosylated peptide, two glycosylated forms of somatostatin-22 were accessed by incorporating as building blocks, respectively, Nalpha-Fmoc-Thr(Ac3-alpha-D-GalNAc)-OH and Nalpha-Fmoc-Thr(Ac4-beta-D-Gal-(1-->3)-Ac2-alpha-D-GalNAc)-O H. Acidolytic deprotection/cleavage of these peptidyl-resins with trifluoroacetic acid/scavenger cocktails gave the corresponding acetyl-protected glycopeptides with free sulfhydryl functions. Deacetylation, by methanolysis in the presence of catalytic sodium methoxide, was followed by mild oxidation at pH 7, mediated by Nalpha-dithiasuccinoyl (Dts)-glycine, to provide the desired monomeric cyclic disulfides. The purified peptides were tested for binding affinities to a panel of cloned human somatostatin receptor subtypes; in several cases, presence of the disaccharide moiety resulted in 2-fold tighter binding.
Stereoselective Polymer-Supported Synthesis of Morpholine- and Thiomorpholine-3-carboxylic Acid Derivatives.[Pubmed:28085245]
ACS Comb Sci. 2017 Mar 13;19(3):173-180.
Herein we report the polymer-supported synthesis of 3,4-dihydro-2H-1,4-oxazine-3-carboxylic acid derivatives using immobilized Fmoc-Ser(tBu)-OH and Fmoc-Thr(tBu)-OH as the starting materials. After the solid-phase-synthesis of N-alkyl-N-sulfonyl/acyl intermediates, the target dihydrooxazines were obtained using trifluoroacetic acid-mediated cleavage from the resin. This approach was also studied for the preparation of dihydrothiazines from immobilized Fmoc-Cys(Trt)-OH. Inclusion of triethylsilane in the cleavage cocktail resulted in the stereoselective formation of the corresponding morpholine/thiomorpholine-3-carboxylic acids. Stereochemical studies revealed the specific configuration of the newly formed stereocenter and also the formation of stable N-acylmorpholine rotamers.


