FlunitrazepamActs at the benzodiazepene modulatory site CAS# 1622-62-4 |
- Etifoxine
Catalog No.:BCC1560
CAS No.:21715-46-8
- Etomidate
Catalog No.:BCC1150
CAS No.:33125-97-2
- Etifoxine hydrochloride
Catalog No.:BCC1561
CAS No.:56776-32-0
- Acamprosate calcium
Catalog No.:BCC1327
CAS No.:77337-73-6
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 1622-62-4 | SDF | Download SDF |
| PubChem ID | 3380 | Appearance | Powder |
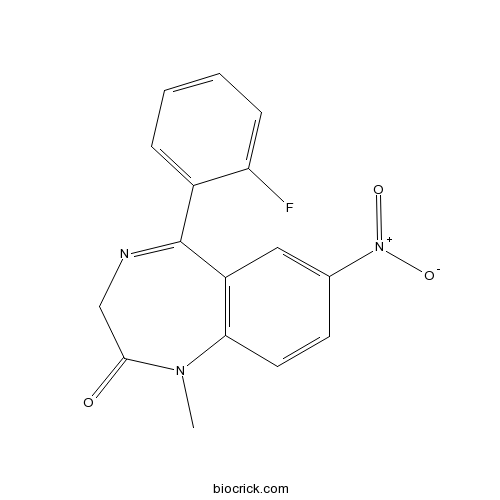
| Formula | C16H12FN3O3 | M.Wt | 313.28 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 10 mM in ethanol | ||
| Chemical Name | 5-(2-fluorophenyl)-1-methyl-7-nitro-3H-1,4-benzodiazepin-2-one | ||
| SMILES | CN1C(=O)CN=C(C2=C1C=CC(=C2)[N+](=O)[O-])C3=CC=CC=C3F | ||
| Standard InChIKey | PPTYJKAXVCCBDU-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C16H12FN3O3/c1-19-14-7-6-10(20(22)23)8-12(14)16(18-9-15(19)21)11-4-2-3-5-13(11)17/h2-8H,9H2,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Ligand at the GABAA receptor benzodiazepine modulatory site. Exhibits hypnotic effects; also displays anxiolytic and sedative properties. |

Flunitrazepam Dilution Calculator

Flunitrazepam Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.192 mL | 15.9602 mL | 31.9203 mL | 63.8407 mL | 79.8008 mL |
| 5 mM | 0.6384 mL | 3.192 mL | 6.3841 mL | 12.7681 mL | 15.9602 mL |
| 10 mM | 0.3192 mL | 1.596 mL | 3.192 mL | 6.3841 mL | 7.9801 mL |
| 50 mM | 0.0638 mL | 0.3192 mL | 0.6384 mL | 1.2768 mL | 1.596 mL |
| 100 mM | 0.0319 mL | 0.1596 mL | 0.3192 mL | 0.6384 mL | 0.798 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- RWJ 50271
Catalog No.:BCC7894
CAS No.:162112-37-0
- SynaptoRedTM C2
Catalog No.:BCC8012
CAS No.:162112-35-8
- 4',4'''-Di-O-methylisochamaejasmin
Catalog No.:BCN6849
CAS No.:1620921-68-7
- Dimesna
Catalog No.:BCC1095
CAS No.:16208-51-8
- Myriceric acid C
Catalog No.:BCN1719
CAS No.:162059-94-1
- SC 58125
Catalog No.:BCC5948
CAS No.:162054-19-5
- L-371,257
Catalog No.:BCC7353
CAS No.:162042-44-6
- UNC0379
Catalog No.:BCC8055
CAS No.:1620401-82-2
- Rofecoxib
Catalog No.:BCC4437
CAS No.:162011-90-7
- Bromosporine
Catalog No.:BCC2226
CAS No.:1619994-69-2
- GSK2801
Catalog No.:BCC6498
CAS No.:1619994-68-1
- LY2857785
Catalog No.:BCC8050
CAS No.:1619903-54-6
- Melperone hydrochloride
Catalog No.:BCC7385
CAS No.:1622-79-3
- 5'-Deoxy-5-fluoro-N-[(pentyloxy)carbonyl]cytidine 2',3'-diacetate
Catalog No.:BCN1544
CAS No.:162204-20-8
- Dorsmanin A
Catalog No.:BCN4088
CAS No.:162229-27-8
- CI 1020
Catalog No.:BCC7523
CAS No.:162256-50-0
- 7,3'-Dihydroxy-4'-methoxyflavan
Catalog No.:BCN4698
CAS No.:162290-05-3
- Remodelin
Catalog No.:BCC5571
CAS No.:1622921-15-6
- Baccatin X
Catalog No.:BCN7214
CAS No.:1623069-76-0
- Oplopanaxoside C
Catalog No.:BCC8226
CAS No.:162341-29-9
- Baccatin VIII
Catalog No.:BCN7212
CAS No.:1623410-10-5
- Baccatin IX
Catalog No.:BCN7213
CAS No.:1623410-12-7
- Hydramicromelin D
Catalog No.:BCN7548
CAS No.:1623437-86-4
- 1-Octanone,1-[4-[2-(acetyloxy)ethyl]phenyl]
Catalog No.:BCN2251
CAS No.:162358-03-4
Prevalence and clinical correlates of flunitrazepam-related complex sleep behaviors.[Pubmed:27778423]
Psychiatry Clin Neurosci. 2017 Mar;71(3):198-203.
AIM: Complex sleep behaviors (CSB) are often associated with the use of hypnotic drugs. This study investigated the prevalence and correlates of CSB among psychiatric patients who were given Flunitrazepam. METHODS: From June 2011 to May 2012, a total of 268 psychiatric outpatients who had received Flunitrazepam for at least 3 months were enrolled. Data on occurrence of CSB, demographic characteristics, Flunitrazepam dosage and duration of use, psychiatric diagnoses, physical illnesses, and alcohol use were collected. Logistic regression analysis was used to examine the clinical correlates of CSB. RESULTS: Sixty-six participants (24.6%) reported experiencing CSB. Logistic regression analysis showed that a high dosage (>2 mg/day) of Flunitrazepam (odds ratio [OR] = 1.941, 95% confidence interval [CI] = 1.090-3.455, P = 0.024) and alcohol use (OR = 1.948, 95%CI = 1.023-3.709, P = 0.042) were significantly associated with the occurrence of CSB. Sex, age, duration of Flunitrazepam use, psychiatric diagnoses, and physical illnesses were not significantly associated with the occurrence of CSB. CONCLUSION: CSB among Flunitrazepam users should be monitored routinely, especially among those receiving a high dosage who also consume alcohol.
Behavioral pharmacology of the odor span task: Effects of flunitrazepam, ketamine, methamphetamine and methylphenidate.[Pubmed:27747877]
J Exp Anal Behav. 2016 Nov;106(3):173-194.
The Odor Span Task is an incrementing non-matching-to-sample procedure that permits the study of behavior under the control of multiple stimuli. Rats are exposed to a series of odor stimuli and selection of new stimuli is reinforced. Successful performance thus requires remembering which stimuli have previously been presented during a given session. This procedure has been frequently used in neurobiological studies as a rodent model of working memory; however, only a few studies have examined the effects of drugs on performance in this task. The present experiments explored the behavioral pharmacology of a modified version of the Odor Span Task by determining the effects of stimulant drugs methylphenidate and methamphetamine, NMDA antagonist ketamine, and positive GABAA modulator Flunitrazepam. All four drugs produced dose-dependent impairment of performances on the Odor Span Task, but for methylphenidate and methamphetamine, these occurred only at doses that had similar effects on performance of a simple odor discrimination. Generally, these disruptions were based on omission of responding at the effective doses. The effects of ketamine and Flunitrazepam were more selective in some rats. That is, some rats tested under Flunitrazepam and ketamine showed decreases in accuracy on the Odor Span Task at doses that did not affect simple discrimination performance. These selective effects indicate disruption of within-session stimulus control. Overall, these findings support the potential of the Odor Span Task as a baseline for the behavioral pharmacological analysis of remembering.
The Effect of Flunitrazepam (Rohypnol((R)) ) on the Development of Chrysomya megacephala (Fabricius, 1794) (Diptera: Calliphoridae) and its Implications for Forensic Entomology.[Pubmed:27143233]
J Forensic Sci. 2016 Jul;61(4):1112-5.
This study investigated the potential effects of Flunitrazepam (known as "date rape drug") on the developmental cycle of Chrysomya megacephala, an important forensic species, and their possible implications for the calculation of the PMI. A 1050 C. megacephala eggs were divided into five groups with seven replications each. The eggs were placed on artificial diet prepared with four drug concentrations of Flunitrazepam (4, 8, 16, and 32 ng/g), besides the control group (prepared with water). Were evaluated the potential effects on development time, weight gain, and mortality during the cycles. The drug had no significant effect on development time or mortality although it did affect the weight of the pupae and adults (Kruskal-Wallis, p < 0.05). The result can be deduced that the determination of the postmortem interval is not affected.
The Importance of L-Arginine:NO:cGMP Pathway in Tolerance to Flunitrazepam in Mice.[Pubmed:27957675]
Neurotox Res. 2017 Feb;31(2):309-316.
The goal of the study was to investigate the effects of drugs modifying L-arginine:NO:cGMP pathway on the development of tolerance to Flunitrazepam (FNZ)-induced motor impairment in mice. FNZ-induced motor incoordination was assessed on the 1st and 8th days of experiment, using the rotarod and chimney tests. It was found that (a) both a non-selective nitric oxide synthase (NOS) inhibitor: N (G)-nitro-L-arginine methyl ester (L-NAME) and an unselective neuronal NOS inhibitor: 7-nitroindazole (7-NI) inhibited the development of tolerance to the motor-impairing effects of FNZ in the rotarod and the chimney tests and (b) both a NO precursor: L-arginine and a selective inhibitor of phosphodiesterase 5 (PDE5): sildenafil did not affect the development of tolerance to FNZ-induced motor impairment in mice. Those findings provided behavioural evidence that NO could contribute an important role in the development of tolerance to FNZ in mice.
Flunitrazepam rapidly reduces GABA(A) receptor subunit protein expression via a protein kinase C-dependent mechanism.[Pubmed:9723942]
Br J Pharmacol. 1998 Aug;124(7):1338-40.
Acute Flunitrazepam (1 microM) exposure for 1 h reduced GABA(A) receptor alpha1 (22+/-4%, mean+/-s.e.mean) and beta2/3 (21+/-4%) subunit protein levels in cultured rat cerebellar granule cells. This rapid decrease in subunit proteins was completely prevented by bisindolymaleimide 1 (1 microM), an inhibitor of protein kinase C, but not by N-[2-((p-bromocinnamyl)amino)ethyl]-5-isoquinolinesulfonamide (H-89, 4.8 microM), an inhibitor of protein kinases A and G. These results suggest the existence of a benzodiazepine-induced mechanism to rapidly alter GABA(A) receptor protein expression, that appears to be dependent on protein kinase C activity.


