Flavoxate hydrochlorideCAS# 3717-88-2 |
- LY2835219
Catalog No.:BCC1113
CAS No.:1231930-82-7
- Roscovitine (Seliciclib,CYC202)
Catalog No.:BCC1105
CAS No.:186692-46-6
- Nu 6027
Catalog No.:BCC1154
CAS No.:220036-08-8
- SNS-032 (BMS-387032)
Catalog No.:BCC1152
CAS No.:345627-80-7
- AT7519 Hydrochloride
Catalog No.:BCC1376
CAS No.:902135-91-5
Quality Control & MSDS
Number of papers citing our products

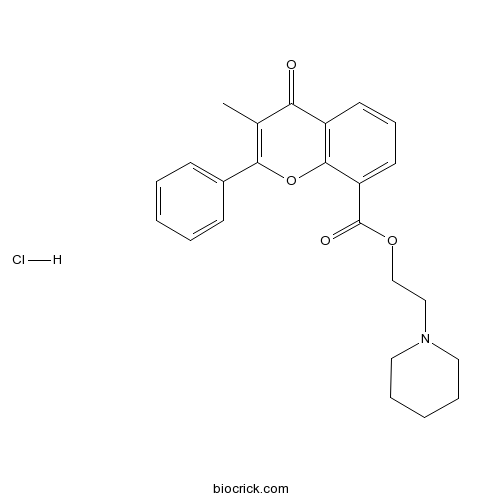
Chemical structure
3D structure
| Cas No. | 3717-88-2 | SDF | Download SDF |
| PubChem ID | 441345 | Appearance | Powder |
| Formula | C24H26ClNO4 | M.Wt | 427.92 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Rec-7-0040; DW61 | ||
| Solubility | H2O : 5 mg/mL (11.68 mM; Need ultrasonic) DMSO : 3.33 mg/mL (7.78 mM; Need ultrasonic) | ||
| Chemical Name | 2-piperidin-1-ylethyl 3-methyl-4-oxo-2-phenylchromene-8-carboxylate;hydrochloride | ||
| SMILES | CC1=C(OC2=C(C1=O)C=CC=C2C(=O)OCCN3CCCCC3)C4=CC=CC=C4.Cl | ||
| Standard InChIKey | XOEVKNFZUQEERE-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C24H25NO4.ClH/c1-17-21(26)19-11-8-12-20(23(19)29-22(17)18-9-4-2-5-10-18)24(27)28-16-15-25-13-6-3-7-14-25;/h2,4-5,8-12H,3,6-7,13-16H2,1H3;1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Flavoxate Hydrochloride(DW-61 Hydrochloride) is a muscarinic AChR antagonist used in various urinary syndromes and as an antispasmodic.
Target: mAChR
Flavoxate displaces [3H]nitrendipine on the Ca2+ channels binding sites with IC50 of 254 μM [1]. Flavoxate (>10 μM) suppresses carbachol-induced contractions in isolated rat detrusor strips with pD value of 4.55. Flavoxate (>10 μM) suppresses Ca2+-induced contractions in isolated rat detrusor strips with pIC50 value of 4.92 [2]. Flavoxate (0.01 μM ?10 μM) inhibits CAMP formation in a concentration-dependent manner in membranes from the rat striatum and cerebral cortex, an action which is completely abolished by pretreating the membranes with pertussis toxin (PTX) [3].
Flavoxate (10mg/kg) suppresses both the an initial, rapidly rising phasic contraction (phase 1) and the tonic contraction (phase 2) contractions to the same extent in rats. Flavoxate (10mg/kg) abolishes the bladder contractions without causing any change in the amplitude of the contractions in rats. Flavoxate (3 mg/kg) abolishes the efferent neural activity and the associated bladder contractions for about 10 minutes without changing the baseline vesical pressure in rats. ICV-injected (50 to 200 μg/rat) or IT-injected (100 to 200 μg/rat) Flavoxate abolishes rhythmic bladder contractions during and after injection for five to 15 minutes in a dose-dependent manner in rats [2]. Flavoxate (3 mg/kg, i.v.) abolishes rhythmic bladder contractions and the maximal intervals of voiding contractions is 7.20 min [3]. References: | |||||

Flavoxate hydrochloride Dilution Calculator

Flavoxate hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3369 mL | 11.6844 mL | 23.3689 mL | 46.7377 mL | 58.4221 mL |
| 5 mM | 0.4674 mL | 2.3369 mL | 4.6738 mL | 9.3475 mL | 11.6844 mL |
| 10 mM | 0.2337 mL | 1.1684 mL | 2.3369 mL | 4.6738 mL | 5.8422 mL |
| 50 mM | 0.0467 mL | 0.2337 mL | 0.4674 mL | 0.9348 mL | 1.1684 mL |
| 100 mM | 0.0234 mL | 0.1168 mL | 0.2337 mL | 0.4674 mL | 0.5842 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Flavoxate Hydrochloride(DW-61 Hydrochloride) is a muscarinic AChR antagonist used in various urinary syndromes and as an antispasmodic.
- 4'-Amino-3',5'-dichloroacetophenone
Catalog No.:BCC8678
CAS No.:37148-48-4
- Murrangatin
Catalog No.:BCN5426
CAS No.:37126-91-3
- IC-87114
Catalog No.:BCC1161
CAS No.:371242-69-2
- Azlocillin sodium salt
Catalog No.:BCC4763
CAS No.:37091-65-9
- Procyanidin C1
Catalog No.:BCN6317
CAS No.:37064-30-5
- Z-Glu-OBzl
Catalog No.:BCC2780
CAS No.:3705-42-8
- Cyclo(Gly-L-Pro)
Catalog No.:BCN4059
CAS No.:3705-27-9
- Cyclo(L-Phe-L-Pro)
Catalog No.:BCN4029
CAS No.:3705-26-8
- Carbetocin
Catalog No.:BCC6304
CAS No.:37025-55-1
- (-)-Variabilin
Catalog No.:BCN4815
CAS No.:370102-93-5
- Zinterol hydrochloride
Catalog No.:BCC6911
CAS No.:37000-20-7
- FCCP
Catalog No.:BCC5659
CAS No.:370-86-5
- PI-103
Catalog No.:BCC1162
CAS No.:371935-74-9
- PI-103 Hydrochloride
Catalog No.:BCC1860
CAS No.:371935-79-4
- YM201636
Catalog No.:BCC4996
CAS No.:371942-69-7
- ZAPA sulfate
Catalog No.:BCC6563
CAS No.:371962-01-5
- SB 612111 hydrochloride
Catalog No.:BCC7714
CAS No.:371980-94-8
- L-Citruline
Catalog No.:BCN2692
CAS No.:372-75-8
- Capsidiol
Catalog No.:BCC8140
CAS No.:37208-05-2
- Citromycin
Catalog No.:BCN7459
CAS No.:37209-30-6
- PIK-75
Catalog No.:BCC1163
CAS No.:372196-77-5
- Sieber Linker
Catalog No.:BCC2835
CAS No.:3722-51-8
- Wilforgine
Catalog No.:BCN5427
CAS No.:37239-47-7
- Wilfortrine
Catalog No.:BCN3085
CAS No.:37239-48-8
[Fluorescence enhancement of flavoxate hydrochloride in alkali solution and its application in pharmaceutical analysis].[Pubmed:26837181]
Yao Xue Xue Bao. 2015 Oct;50(10):1324-9.
Fluorescence enhancement reaction of Flavoxate hydrochloride (FX) in strong alkali solution was studied, the mechanism of the reaction was investigated, and a novel fluorimetric method for analysis of FX in drug sample was established. FX has no intrinsic fluorescence, but it can slowly produce fluorescence in strong alkali solution. Heating can promote the fluorescence enhancement reaction. In 3D fluorescence spectra of the decomposition product of FX, two fluorescence peaks, located respectively at excitation wavelengths lambdaex/ emission wavelength lambdaem =223/410 nm, and 302/410 nm, were observed. Using quinine sulfate as a reference, fluorescence quantum yield of the decomposition product was measured to be 0.50. The structural characteriza- tion and spectral analysis of the decomposition product reveal that ester bond hydrolysis reaction of FX is firstly occurred during heating process, forming 3-methylflavone-8-carboxylic acid (MFA), then a cleavage reaction of the gamma-pyrone ring of MFA occurred, producing alpha, beta-unsaturated ketone. This product includes adjacent hydroxyl benzoic acid group in its molecule, which can form intramolecular hydrogen bond under alkaline condition, so that increase the conjugate degree and enhance the rigidity of the molecule, and thereby cause fluorescence enhancement. Based on this fluorescence enhancement reaction, a fluorimetric method was proposed for the determination of FX. A linear calibration curve covered the concentration range 0.020 3-0.487 microg . mL. The regression equation was I(F) = 23.9 + 5357.3 c, with correlation coefficient r = 0.999 7 (n = 8), detection limit D = 1.1 ng . mL(-1). The method was applied to the analysis of FX tablets, with a spiked recovery rate of 100.2%. The reliability of the method was verified by a UV-spectrophotometric method.
Spectrofluorimetric determination of 3-methylflavone-8-carboxylic acid, the main active metabolite of flavoxate hydrochloride in human urine.[Pubmed:25004902]
Spectrochim Acta A Mol Biomol Spectrosc. 2015 Jan 5;134:109-13.
A simple, sensitive and selective spectrofluorimetric method has been developed for the determination of 3-methylflavone-8-carboxylic acid as the main active metabolite of Flavoxate hydrochloride in human urine. The proposed method was based on the measurement of the native fluorescence of the metabolite in methanol at an emission wavelength 390 nm, upon excitation at 338 nm. Moreover, the urinary excretion pattern has been calculated using the proposed method. Taking the advantage that 3-methylflavone-8-carboxylic acid is also the alkaline degradate, the proposed method was applied to in vitro determination of Flavoxate hydrochloride in tablets dosage form via the measurement of its corresponding degradate. The method was validated in accordance with the ICH requirements and statistically compared to the official method with no significant difference in performance.
Simultaneous Determination of Ofloxacin and Flavoxate Hydrochloride by Absorption Ratio and Second Derivative UV Spectrophotometry.[Pubmed:24826003]
J Basic Clin Pharm. 2010 Dec;2(1):53-61. Epub 2011 Feb 15.
The objective of this study was to develop simple, precise, accurate and sensitive UV spectrophotometric methods for the simultaneous determination of ofloxacin (OFX) and flavoxate HCl (FLX) in pharmaceutical formulations. The first method is based on absorption ratio method, by formation of Q absorbance equation at 289 nm (lambdamax of OFX) and 322.4 nm (isoabsorptive point). The linearity range was found to be 1 to 30 mug/ml for FLX and OFX. In the method-II second derivative absorption at 311.4 nm for OFX (zero crossing for FLX) and at 246.2 nm for FLX (zero crossing for OFX) was used for the determination of the drugs and the linearity range was found to be 2 to 30 mug/ml for OFX and 2-75 mug /ml for FLX. The accuracy and precision of the methods were determined and validated statistically. Both the methods showed good reproducibility and recovery with % RSD less than 1.5%. Both the methods were found to be rapid, specific, precise and accurate and can be successfully applied for the routine analysis of OFX and FLX in combined dosage form.
Polymeric matrix membrane sensors for stability-indicating potentiometric determination of oxybutynin hydrochloride and flavoxate hydrochloride urogenital system drugs.[Pubmed:19202792]
J AOAC Int. 2008 Nov-Dec;91(6):1318-30.
Four polyvinyl chloride (PVC) matrix membrane electrodes responsive to 2 drugs affecting the urogenital system--oxybutynin hydrochloride (OX) and Flavoxate hydrochloride (FX)--were developed, described, and characterized. A precipitation-based technique with tungstophosphate (TP) and ammonium reineckate (R) anions as electroactive materials in a PVC matrix with an OX cation was used for electrode 1 and 2 fabrication, respectively. Electrode 3 and 4 fabrication was based on use of the precipitation technique of FX cation with tetrakis (4-chlorophenyl) borate and R anions as electroactive materials. Fast and stable Nernstian responses in the range 1 x 10(-2)-1 x 10(-6) M for the 2 drugs over the pH range 5-8 revealed the performance characteristics of these electrodes, which were evaluated according to International Union of Pure and Applied Chemistry recommendations. The method was applied to FX and OX in their pharmaceutical formulations and in human plasma samples. The 4 proposed sensors were found to be specific for the drugs in the presence of up to 60% of their degradation products. Validation of the method according to the quality assurance standards showed suitability of the proposed electrodes for use in the quality control assessment of these drugs. The recoveries for determination of the drugs by the 4 proposed selective electrodes were 99.5 +/- 0.5, 100.0 +/- 0.4, 99.9 +/- 0.4, and 100.1 +/- 0.4% for sensors 1-4, respectively. Statistical comparison between the results obtained by this method and the official method of the drugs was done, and no significant difference found.


