EurocristatineCAS# 1459722-08-7 |
Quality Control & MSDS
Number of papers citing our products

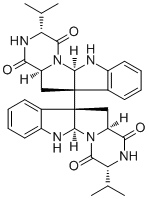
Chemical structure

| Cas No. | 1459722-08-7 | SDF | Download SDF |
| PubChem ID | N/A | Appearance | Powder |
| Formula | C32H36N6O4 | M.Wt | 568.74 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Eurocristatine Dilution Calculator

Eurocristatine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.7583 mL | 8.7914 mL | 17.5827 mL | 35.1655 mL | 43.9568 mL |
| 5 mM | 0.3517 mL | 1.7583 mL | 3.5165 mL | 7.0331 mL | 8.7914 mL |
| 10 mM | 0.1758 mL | 0.8791 mL | 1.7583 mL | 3.5165 mL | 4.3957 mL |
| 50 mM | 0.0352 mL | 0.1758 mL | 0.3517 mL | 0.7033 mL | 0.8791 mL |
| 100 mM | 0.0176 mL | 0.0879 mL | 0.1758 mL | 0.3517 mL | 0.4396 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 1-O-Caffeoyl-3,4-di-O-galloyl-β-D-glucopyranose
Catalog No.:BCX0534
CAS No.:359819-46-8
- 13-Hydroxynootkatone
Catalog No.:BCX0533
CAS No.:141695-86-5
- 1β,8α-Dihydroxyeremophila-7(11),9-dien-12,8-olide
Catalog No.:BCX0532
CAS No.:849700-44-3
- Salcolin A
Catalog No.:BCX0531
CAS No.:1977557-69-9
- 2-Hydroxymethyl-5-isopropylphenol
Catalog No.:BCX0530
CAS No.:111044-81-6
- 4-Hydroxythonningianin B
Catalog No.:BCX0529
CAS No.:2329726-95-4
- 5,7,4'-Trihydroxy-3,8-dimethoxyflavone
Catalog No.:BCX0528
CAS No.:14965-09-4
- 5-(1-Hydroxypropan-2-yl)-2-methylphenol
Catalog No.:BCX0527
CAS No.:111044-80-5
- (E)-5-Hydroxy-6-isoprenyl-2-(pent-1-en-1-yl)benzofuran-4-carbaldehyde
Catalog No.:BCX0526
CAS No.:916602-30-7
- Dihydroauroglaucin
Catalog No.:BCX0525
CAS No.:77102-91-1
- Butyl rosmarinate
Catalog No.:BCX0524
CAS No.:222713-83-9
- Labda-12E,14-dien-16,15-olid-17-oic acid
Catalog No.:BCX0523
CAS No.:1855905-16-6
- Maltol 3-O-(5-O-p-coumaroyl)-β-D-apiofuranosyl-(1→6)-O-β-D-glucopyranoside
Catalog No.:BCX0536
CAS No.:1018452-31-7
- Variecolorin G
Catalog No.:BCX0537
CAS No.:957780-76-6
- Physcion-10,10'-bianthrone
Catalog No.:BCX0538
CAS No.:21871-90-9
- 22-Hydroxyhopan-2-one
Catalog No.:BCX0539
CAS No.:97230-81-4
- 4-Hydroxythonningianin A
Catalog No.:BCX0540
CAS No.:1055032-77-3
- Catenarin
Catalog No.:BCX0541
CAS No.:476-46-0
- Clemoarmanoside B
Catalog No.:BCX0542
CAS No.:915314-08-8
- Sanggenol H
Catalog No.:BCX0543
CAS No.:202526-53-2
- 9''-O-Acetylsalcolin A
Catalog No.:BCX0544
CAS No.:910864-92-5
- 1β-Hydroxy-12-noreremophila-6,9-diene-8,11-dione
Catalog No.:BCX0545
CAS No.:161127-52-2
- (-)-Lyoniresinol 9-O-glucoside
Catalog No.:BCX0546
CAS No.:162613-63-0
- Isophthalic acid
Catalog No.:BCX0547
CAS No.:121-91-5
Neoechinulin A as a Promising SARS-CoV-2 M(pro) Inhibitor: In Vitro and In Silico Study Showing the Ability of Simulations in Discerning Active from Inactive Enzyme Inhibitors.[Pubmed:35323462]
Mar Drugs. 2022 Feb 24;20(3):163.
The COVID-19 pandemic and its continuing emerging variants emphasize the need to discover appropriate treatment, where vaccines alone have failed to show complete protection against the new variants of the virus. Therefore, treatment of the infected cases is critical. This paper discusses the bio-guided isolation of three indole diketopiperazine alkaloids, neoechinulin A (1), echinulin (2), and Eurocristatine (3), from the Red Sea-derived Aspergillus fumigatus MR2012. Neoechinulin A (1) exhibited a potent inhibitory effect against SARS-CoV-2 M(pro) with IC(50) value of 0.47 muM, which is comparable to the reference standard GC376. Despite the structural similarity between the three compounds, only 1 showed a promising effect. The mechanism of inhibition is discussed in light of a series of extensive molecular docking, classical and steered molecular dynamics simulation experiments. This paper sheds light on indole diketopiperazine alkaloids as a potential structural motif against SARS-CoV-2 M(pro). Additionally, it highlights the potential of different molecular docking and molecular dynamics simulation approaches in the discrimination between active and inactive structurally related M(pro) inhibitors.
Eurocristatine, a plant alkaloid from Eurotium cristatum, alleviates insulin resistance in db/db diabetic mice via activation of PI3K/AKT signaling pathway.[Pubmed:32946868]
Eur J Pharmacol. 2020 Nov 15;887:173557.
Eurocristatine (ECT) is an alkaloid isolated from Eurotium cristatum, and it has been used in multiple applications. However, its use as a treatment for type 2 diabetes mellitus (T2DM) has not yet been reported. In this study, we investigated the anti-T2DM effect of ECT and explored its potential molecular mechanism. In vivo, after treatment with ECT (20, 40 mg/kg) for 6 weeks, fasting blood glucose (FBG) was remarkably reduced in db/db mice. Moreover, glucose tolerance, insulin sensitivity and hyperinsulinemia were ameliorated treatment with ECT. The values of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) also showed that ECT could alleviate liver toxicity caused by diabetes in db/db mice. In vitro, ECT (15 and 30 muM) alleviated insulin resistance by increasing glucose consumption, glucose uptake and glycogen content in high glucose-induced HepG2 cells. The Western blotting (WB) results showed that ECT could upregulate the expression of phosphatidylinositol 3-kinase (PI3K), increase the phosphorylation of insulin receptor substrate 1 (IRS1) and protein kinase B (AKT) in vivo and in vitro. Besides, ECT improved the glycogen content by inhibiting the expression of glycogen synthase kinase3beta (GSK3beta) and promoting that of glycogen synthase (GS). Furthermore, administration of the PI3K/AKT signaling pathway inhibitor LY294002 abolished the beneficial effects of ECT. These findings are the first to verify that ECT has the potential to improve glucose metabolism and alleviate insulin resistance by activating the PI3K/AKT signaling pathway in db/db mice.
Purification, characterization, and hypoglycemic properties of eurocristatine from Eurotium cristatum spores in Fuzhuan brick tea.[Pubmed:35516628]
RSC Adv. 2020 Jun 10;10(37):22234-22241.
Fuzhuan brick tea (FBT) is a Chinese dark tea that is famous for its significant health benefits, in which Eurotium cristatum (E. cristatum) strains play a vital role in its postfermentation process. In this study, Eurocristatine with hypoglycemic activity was discovered for the first time and purified from the spores of E. cristatum growing in FBT. Eurocristatine (98%) was obtained by D-101 macroporous resin-based column chromatography and preparative high performance liquid chromatography (HPLC) with a C(18) column as the stationary phase and 35% acetonitrile in ultrapure water as the mobile phase. Hypoglycemic activity in a Hep-G2 cell hypoglycemic model was used as a screening indicator during purification. The chemical structure of Eurocristatine was characterized by ESI/MS, (1)H NMR and (13)C NMR analyses. The antidiabetic effects of Eurocristatine were verified in high-fat diet/streptozocin-induced type 2 diabetes mellitus (T2DM) rats. The results showed that Eurocristatine significantly reduced fasting blood glucose. Our study demonstrated that Eurocristatine, as a newly discovered hypoglycemic active substance, could be considered a potential candidate for the treatment of diabetes and its complications.
Antibacterial and antibiofilm activities of the metabolites isolated from the culture of the mangrove-derived endophytic fungus Eurotium chevalieri KUFA 0006.[Pubmed:28586721]
Phytochemistry. 2017 Sep;141:86-97.
Five previously undescribed metabolites, including acetylquestinol, two prenylated indole 3-carbaldehyde derivatives, an anthranilic acid derivative and an isochromone derivative, were isolated, in addition to eleven known compounds: palmitic acid, ergosterol 5,8-endoperoxide, emodin, physcion, questin, questinol, (11S, 14R)-cyclo(tryptophylvalyl), preechinulin, neoechinulin E, echinulin and Eurocristatine, from the culture of the endophytic fungus Eurotium chevalieri KUFA 0006. The structures of the previously undescribed compounds were established based on an extensive 1D and 2D NMR spectral analysis as well as HRMS and IR data. In case of 2-(2, 2-dimethylcyclopropyl)-1H-indole-3-carbaldehyde and 6, 8-dihydroxy-3-(2S-hydroxypropyl)-7-methylisochromone, the absolute configurations of their stereogenic carbons were established based on comparison of their experimental and calculated ECD spectra. All the compounds, except for palmitic acid and ergosterol 5, 8-endoperoxide, were evaluated for their antibacterial and antibiofilm activities against two Gram-positive and two Gram-negative bacteria, as well as multidrug-resistant isolates from the environment. Emodin not only exhibited moderate antibacterial activity against the Gram-positive bacteria but also showed strong synergistic association with oxacillin against MRSA Staphylococcus aureus.
Collective Synthesis and Biological Evaluation of Tryptophan-Based Dimeric Diketopiperazine Alkaloids.[Pubmed:26598794]
Chemistry. 2016 Jan 22;22(4):1277-91.
A concise two one-pot synthesis of WIN 64821, Eurocristatine, 15,15'-bis-epi-Eurocristatine, ditryptophenaline, ditryptoleucine A, WIN 64745, cristatumin C, asperdimin, naseseazine A, and naseseazine B is detailed, based on a unique bioinspired dimerization reaction of tryptophan derivatives in aqueous acidic solution and a one-pot procedure for the construction of diketopiperazine rings. Total yields of these alkaloid syntheses were from 10 up to 27 %. In addition, 1'-(2-phenylethylene)-ditryptophenaline was synthesized by using three one-pot operations. The studies detailed herein provided synthesized natural products for inhibitory activities of ubiquitin-specific protease 7 (USP7) and foam cell formation in macrophages. The newly listed biological evaluation for tryptophan-based dimeric diketopiperazine alkaloids discovered 15,15'-bis-epi-Eurocristatine, 1'-(2-phenylethylene)-ditryptophenaline, and WIN 64745 as new drug candidates.


