ErlotinibEGFR tyrosine kinase inhibitor CAS# 183321-74-6 |
- Dacomitinib (PF299804, PF299)
Catalog No.:BCC3683
CAS No.:1110813-31-4
- AG-1478
Catalog No.:BCC3717
CAS No.:153436-53-4
- OSI-420
Catalog No.:BCC4472
CAS No.:183320-51-6
- Butein
Catalog No.:BCN5592
CAS No.:487-52-5
- Neratinib (HKI-272)
Catalog No.:BCC3685
CAS No.:698387-09-6
- AST-1306
Catalog No.:BCC3727
CAS No.:897383-62-9
Quality Control & MSDS
Number of papers citing our products

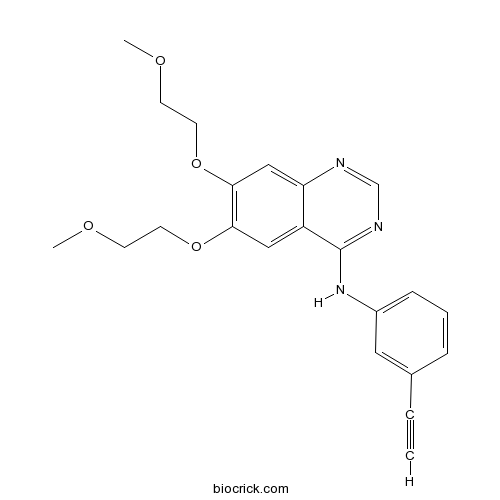
Chemical structure

3D structure
| Cas No. | 183321-74-6 | SDF | Download SDF |
| PubChem ID | 176870 | Appearance | Powder |
| Formula | C22H23N3O4 | M.Wt | 393.44 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | NSC 718781; OSI-744; R1415 | ||
| Solubility | >19.7mg/mL in DMSO | ||
| Chemical Name | N-(3-ethynylphenyl)-6,7-bis(2-methoxyethoxy)quinazolin-4-amine | ||
| SMILES | COCCOC1=C(C=C2C(=C1)C(=NC=N2)NC3=CC=CC(=C3)C#C)OCCOC | ||
| Standard InChIKey | AAKJLRGGTJKAMG-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C22H23N3O4/c1-4-16-6-5-7-17(12-16)25-22-18-13-20(28-10-8-26-2)21(29-11-9-27-3)14-19(18)23-15-24-22/h1,5-7,12-15H,8-11H2,2-3H3,(H,23,24,25) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Erlotinib inhibits purified EGFR kinase with an IC50 of 2 nM.In Vitro:Erlotinib (CP-358,774) is also a potent inhibitor of the recombinant intracellular (kinase) domain of the EGFR, with an IC50 of 1 nM. The proliferation of DiFi cells is strongly inhibited by Erlotinib with an IC50 of 100 nM for an 8-day proliferation assay[1]. The combination of B-DIM and Erlotinib (2 μM) results in a significant inhibition of colony formation in BxPC-3 cells when compared with either agent alone. The combination of B-DIM and Erlotinib (2 μM) results in a significant induction of apoptosis only in BxPC-3 cells when compare with the apoptotic effect of either agent alone[2].In Vivo:Under the experimental conditions, the combination of B-DIM and Erlotinib (50 mg/kg, i.p.) treatment shows significant decrease (P <0.01) in tumor weight compared with untreated control[2]. Erlotinib (20 mg/kg, p.o.) significantly attenuates Cisplatin (CP)-induced body weight (BW) loss when compared to the CP+vehicle (V) rats (P<0.05). Erlotinib treatment significantly improves renal function in CP-N(normal control group, NC) rats. The CP+Erlotinib (E) rats show significant reduction of the levels of Serum creatinine (s-Cr) (P<0.05), blood urea nitrogen (BUN) (P<0.05), urinary N-acetyl-β-D-glucosaminidase (NAG) index (P<0.05), and significant increase of urine volume (UV) (P<0.05) and Cr clearance (Ccr) (P<0.05) compare to the CP+V rats[3] References: | |||||

Erlotinib Dilution Calculator

Erlotinib Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5417 mL | 12.7084 mL | 25.4168 mL | 50.8337 mL | 63.5421 mL |
| 5 mM | 0.5083 mL | 2.5417 mL | 5.0834 mL | 10.1667 mL | 12.7084 mL |
| 10 mM | 0.2542 mL | 1.2708 mL | 2.5417 mL | 5.0834 mL | 6.3542 mL |
| 50 mM | 0.0508 mL | 0.2542 mL | 0.5083 mL | 1.0167 mL | 1.2708 mL |
| 100 mM | 0.0254 mL | 0.1271 mL | 0.2542 mL | 0.5083 mL | 0.6354 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Erlotinib (also known as NSC 718781 or CP 358,774) is a potent and orally-bioavailable inhibitor of epidermal growth factor receptor (EGFR) tyrosine kinase that selectively and reversibly inhibits EGFR-associated intracellular autophosphorylation of tyrosine kinase. Erlotinib inhibits purified EGFR tyrosine kinase and EGFR autophosphorylation intact cells with 50% inhibition concentration IC50 values of 2 nmol/L and 20 nmol/L respectively. Erlotinib competes for the ATP-binding sits on the intracellular domain of EGFR resulting in the inhibition of downstream signaling pathway involved in angiogenesis, cell propagation and cell survival. Erlotinib concentration-dependently inhibits EGFR-mediated propagation signals transduction, displays prominent anti-tumor activity against neoplasms harboring EGFR expression and exhibits a tolerable toxicologic profile.
Reference
Janine Smith. Erlotinib: small-molecule targeted therapy in the treatment of non-small-cell lung cancer. Clinical Therapeutics 2005; 27(10): 1513-1534
- OSI-420
Catalog No.:BCC4472
CAS No.:183320-51-6
- Erlotinib Hydrochloride
Catalog No.:BCC3645
CAS No.:183319-69-9
- 5-(1-Piperazinyl)benzofuran-2-carboxamide
Catalog No.:BCC8717
CAS No.:183288-46-2
- 2-Acetoxy-3-deacetoxycaesaldekarin E
Catalog No.:BCN7476
CAS No.:18326-06-2
- AM251
Catalog No.:BCC4412
CAS No.:183232-66-8
- 1,7-Dihydroxy-2,3-methylenedioxyxanthone
Catalog No.:BCN7543
CAS No.:183210-63-1
- Tipiracil hydrochloride
Catalog No.:BCC2001
CAS No.:183204-72-0
- Guanylin (human)
Catalog No.:BCC7204
CAS No.:183200-12-6
- Cabazitaxel
Catalog No.:BCC4966
CAS No.:183133-96-2
- Fabiatrin
Catalog No.:BCN2920
CAS No.:18309-73-4
- 3,4-O-Isopropylidene shikimic acid
Catalog No.:BCN1147
CAS No.:183075-03-8
- Didrovaltrate
Catalog No.:BCN7124
CAS No.:18296-45-2
- CPPG
Catalog No.:BCC6872
CAS No.:183364-82-1
- Apicidin
Catalog No.:BCC3599
CAS No.:183506-66-3
- Cleroindicin A
Catalog No.:BCC8916
CAS No.:176598-06-4
- CYN 154806
Catalog No.:BCC5823
CAS No.:183658-72-2
- Penthiopyrad
Catalog No.:BCC8072
CAS No.:183675-82-3
- 1,2,3,4,5,6-Hexabromocyclohexane
Catalog No.:BCC2437
CAS No.:1837-91-8
- MRS 1220
Catalog No.:BCC6972
CAS No.:183721-15-5
- Amyloid β-Protein (1-15)
Catalog No.:BCC1003
CAS No.:183745-81-5
- Cyanidin 3-sophoroside chloride
Catalog No.:BCN2611
CAS No.:18376-31-3
- Mithramycin A
Catalog No.:BCC2470
CAS No.:18378-89-7
- Ciproxifan
Catalog No.:BCC4539
CAS No.:184025-18-1
- Ciproxifan maleate
Catalog No.:BCC4049
CAS No.:184025-19-2
MET amplification and epithelial-to-mesenchymal transition exist as parallel resistance mechanisms in erlotinib-resistant, EGFR-mutated, NSCLC HCC827 cells.[Pubmed:28368392]
Oncogenesis. 2017 Apr 3;6(4):e307.
Although many epidermal growth factor receptor (EGFR)-mutated lung cancer patients initially benefit from the EGFR-inhibitor Erlotinib, all acquire resistance. So far, several mechanisms implicated in resistance have been identified, but the existence of multiple resistance mechanisms in parallel have only been sparsely investigated. In this study, we investigated parallel resistance mechanisms acquired by HCC827, an EGFR-mutated adenocarcinoma cell line dependent on EGFR activity and sensitive to Erlotinib. The cell line was treated with Erlotinib by stepwise escalation of the drug-concentration and Erlotinib-resistant (HCC827ER) cells created. HCC827ER cells depicted a mixed epithelial and mesenchymal phenotype. To clarify potential parallel resistance mechanisms, 14 resistant subclones were established by limited dilution. Interestingly, all HCC827ER subclones harbored either a MET-amplification (6/14) or underwent EMT (8/14), mechanisms both found in previous studies, but not in co-occurrence. Both subclone-types were resistant to Erlotinib, but only MET-subclones responded to the MET-inhibitors crizotinib and capmatinib. EMT-subclones on the other hand had markedly increased FGFR1 expression and responded to the FGFR-inhibitor AZD4547, whereas MET-subclones did not. Monitoring gene expression through the development of HCC827ER revealed upregulation of FGFR1 expression as an early response to Erlotinib. In addition, FGFR1 expression increased upon short-term Erlotinib treatment (48 h) identifying a physiological role immediately after Erlotinib exposure. The high FGFR1 expression seen in EMT-subclones was stable even after five passages without Erlotinib. Here we show, that parallel resistance mechanisms appear during Erlotinib-resistance development in EGFR-mutated NSCLC cells and highlight a role for FGFR1 expression changes as an early response to Erlotinib as well as a bypass-signaling mechanism.
A phase Ib/II study of cabozantinib (XL184) with or without erlotinib in patients with non-small cell lung cancer.[Pubmed:28352985]
Cancer Chemother Pharmacol. 2017 May;79(5):923-932.
PURPOSE: Cabozantinib is a multi-kinase inhibitor that targets MET, AXL, and VEGFR2, and may synergize with EGFR inhibition in NSCLC. Cabozantinib was assessed alone or in combination with Erlotinib in patients with progressive NSCLC and EGFR mutations who had previously received Erlotinib. METHODS: This was a phase Ib/II study (NCT00596648). The primary objectives of phase I were to assess the safety, pharmacokinetics, and pharmacodynamics and to determine maximum tolerated dose (MTD) of cabozantinib plus Erlotinib in patients who failed prior Erlotinib treatment. In phase II, patients with prior response or stable disease with Erlotinib who progressed were randomized to single-agent cabozantinib 100 mg qd vs cabozantinib 100 mg qd and Erlotinib 50 mg qd (phase I MTD), with a primary objective of estimating objective response rate (ORR). RESULTS: Sixty-four patients were treated in phase I. Doses of 100 mg cabozantinib plus 50 mg Erlotinib, or 40 mg cabozantinib plus 150 mg Erlotinib were determined to be MTDs. Diarrhea was the most frequent dose-limiting toxicity and the most frequent AE (87.5% of patients). The ORR for phase I was 8.2% (90% CI 3.3-16.5). In phase II, one patient in the cabozantinib arm (N = 15) experienced a partial response, for an ORR of 6.7% (90% CI 0.3-27.9), with no responses for cabozantinib plus Erlotinib (N = 13). There was no evidence that co-administration of cabozantinib markedly altered Erlotinib pharmacokinetics or vice versa. CONCLUSIONS: Despite responses with cabozantinib/Erlotinib in phase I, there were no responses in the combination arm of phase II in patients with acquired resistance to Erlotinib. Cabozantinib did not appear to re-sensitize these patients to Erlotinib.
24h-gene variation effect of combined bevacizumab/erlotinib in advanced non-squamous non-small cell lung cancer using exon array blood profiling.[Pubmed:28359318]
J Transl Med. 2017 Mar 30;15(1):66.
BACKGROUND: The SAKK 19/05 trial investigated the safety and efficacy of the combined targeted therapy bevacizumab and Erlotinib (BE) in unselected patients with advanced non-squamous non-small cell lung cancer (NSCLC). Although activating EGFR mutations were the strongest predictors of the response to BE, some patients not harboring driver mutations could benefit from the combined therapy. The identification of predictive biomarkers before or short after initiation of therapy is therefore paramount for proper patient selection, especially among EGFR wild-types. The first aim of this study was to investigate the early change in blood gene expression in unselected patients with advanced non-squamous NSCLC treated by BE. The second aim was to assess the predictive value of blood gene expression levels at baseline and 24h after BE therapy. METHODS: Blood samples from 43 advanced non-squamous NSCLC patients taken at baseline and 24h after initiation of therapy were profiled using Affymetrix' exon arrays. The 24h gene dysregulation was investigated in the light of gene functional annotations using gene set enrichment analysis. The predictive value of blood gene expression levels was assessed and validated using an independent dataset. RESULTS: Significant gene dysregulations associated with the 24h-effect of BE were detected from blood-based whole-genome profiling. BE had a direct effect on "Pathways in cancer", by significantly down-regulating genes involved in cytokine-cytokine receptor interaction, MAPK signaling pathway and mTOR signaling pathway. These pathways contribute to phenomena of evasion of apoptosis, proliferation and sustained angiogenesis. Other signaling pathways specifically reflecting the mechanisms of action of Erlotinib and the anti-angiogenesis effect of bevacizumab were activated. The magnitude of change of the most dysregulated genes at 24h did not have a predictive value regarding the patients' response to BE. However, predictive markers were identified from the gene expression levels at 24h regarding time to progression under BE. CONCLUSIONS: The 24h-effect of the combined targeted therapy BE could be accurately monitored in advanced non-squamous NSCLC blood samples using whole-genome exon arrays. Putative predictive markers at 24h could reflect patients' response to BE after adjusting for their mutational status. Trial registration ClinicalTrials.gov: NCT00354549.
Niclosamide inhibition of STAT3 synergizes with erlotinib in human colon cancer.[Pubmed:28367059]
Onco Targets Ther. 2017 Mar 23;10:1767-1776.
Niclosamide, an anthelmintic drug approved by the US Food and Drug Administration against cestodes, is used to treat tapeworm infection. In this study, we show that niclosamide can potentially inhibit signal transducer and activator of transcription 3 (STAT3) in colon cancer cell lines. Combined inhibition of epidermal growth factor receptor and STAT3 by Erlotinib and niclosamide synergistically induces apoptosis and antiproliferation in colon cancer cell lines. Our findings suggest that Erlotinib and niclosamide combination provides an effective therapeutic approach to improving the prognosis of colon cancer.


