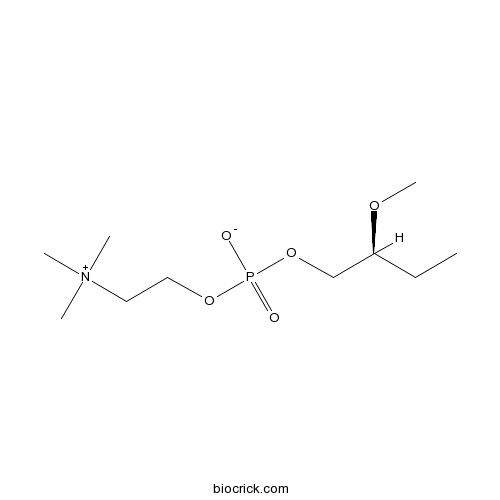
EdelfosineSelective PI-PLC inhibitor, also PAF receptor agonist CAS# 77286-66-9 |
- Ruscogenin
Catalog No.:BCN6287
CAS No.:472-11-7
- Pectolinarigenin
Catalog No.:BCN5813
CAS No.:520-12-7
- Dauricine
Catalog No.:BCN4977
CAS No.:524-17-4
- Oroxin A
Catalog No.:BCN1202
CAS No.:57396-78-8
- Triptophenolide
Catalog No.:BCN2546
CAS No.:74285-86-2
- Wilforlide A
Catalog No.:BCN4383
CAS No.:84104-71-2
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 77286-66-9 | SDF | Download SDF |
| PubChem ID | 7157254 | Appearance | Powder |
| Formula | C27H58NO6P | M.Wt | 523.73 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | ET-18-OCH<sub>3</sub> | ||
| Solubility | Soluble to 5 mM in water with gentle warming and to 100 mM in ethanol | ||
| Chemical Name | [(2S)-2-methoxybutyl] 2-(trimethylazaniumyl)ethyl phosphate | ||
| SMILES | CCC(COP(=O)([O-])OCC[N+](C)(C)C)OC | ||
| Standard InChIKey | GVMCXWJIRSIWFJ-JTQLQIEISA-N | ||
| Standard InChI | InChI=1S/C10H24NO5P/c1-6-10(14-5)9-16-17(12,13)15-8-7-11(2,3)4/h10H,6-9H2,1-5H3/t10-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Synthetic lysophospholipid analog that selectively inhibits phosphatidylinositol phospholipase C (IC50= 9.6 μM in fibroblasts and adenocarcinoma cells). Also acts as an agonist at platelet-activating factor (PAF) receptors. Antitumor lipid; selectively induces apoptosis in tumor cells, sparing normal cells. |

Edelfosine Dilution Calculator

Edelfosine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.9094 mL | 9.5469 mL | 19.0938 mL | 38.1876 mL | 47.7345 mL |
| 5 mM | 0.3819 mL | 1.9094 mL | 3.8188 mL | 7.6375 mL | 9.5469 mL |
| 10 mM | 0.1909 mL | 0.9547 mL | 1.9094 mL | 3.8188 mL | 4.7735 mL |
| 50 mM | 0.0382 mL | 0.1909 mL | 0.3819 mL | 0.7638 mL | 0.9547 mL |
| 100 mM | 0.0191 mL | 0.0955 mL | 0.1909 mL | 0.3819 mL | 0.4773 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Fmoc-Nle-OH.
Catalog No.:BCC3298
CAS No.:77284-32-3
- Zederone
Catalog No.:BCN3524
CAS No.:7727-79-9
- Abyssinone V
Catalog No.:BCN6825
CAS No.:77263-11-7
- Erythrabyssin II
Catalog No.:BCN4828
CAS No.:77263-06-0
- MG 624
Catalog No.:BCC7028
CAS No.:77257-42-2
- Carboxyatractyloside
Catalog No.:BCN2880
CAS No.:77228-71-8
- Nefiracetam
Catalog No.:BCC4504
CAS No.:77191-36-7
- Rheediaxanthone A
Catalog No.:BCN7411
CAS No.:77181-97-6
- Acetylaconitine
Catalog No.:BCN2407
CAS No.:77181-26-1
- Delta-Caesalpin
Catalog No.:BCN6698
CAS No.:7716-14-5
- Spiraline
Catalog No.:BCN2112
CAS No.:77156-25-3
- Spiracine
Catalog No.:BCN2095
CAS No.:77156-24-2
- Hirsutine
Catalog No.:BCN2758
CAS No.:7729-23-9
- SL 0101-1
Catalog No.:BCC8086
CAS No.:77307-50-7
- Hyuganin D
Catalog No.:BCN7679
CAS No.:77331-76-1
- Acamprosate calcium
Catalog No.:BCC1327
CAS No.:77337-73-6
- Triacetylpseurotin A
Catalog No.:BCN6916
CAS No.:77353-57-2
- Drimiopsin C
Catalog No.:BCN4325
CAS No.:773850-90-1
- Drimiopsin D
Catalog No.:BCN4326
CAS No.:773850-91-2
- Sesartemin
Catalog No.:BCN4779
CAS No.:77394-27-5
- Episesartemin A
Catalog No.:BCN7239
CAS No.:77449-31-1
- 5-Androsten-3β-ol-17-one ethyleneketal
Catalog No.:BCC8738
CAS No.:7745-40-6
- KM 11060
Catalog No.:BCC7578
CAS No.:774549-97-2
- Pimobendan hydrochloride
Catalog No.:BCC4175
CAS No.:77469-98-8
Doxorubicin and edelfosine lipid nanoparticles are effective acting synergistically against drug-resistant osteosarcoma cancer cells.[Pubmed:27998763]
Cancer Lett. 2017 Mar 1;388:262-268.
Despite the great advances that have been made in osteosarcoma therapy during recent decades, recurrence and metastases are still the most common outcome of the primary disease. Current treatments include drugs such as doxorubicin (DOX) that produce an effective response during the initial exposure of tumor cells but sometimes induce drug resistance within a few cycles of chemotherapy. New therapeutic strategies are therefore needed to overcome this resistance. To this end, DOX was loaded into lipid nanoparticles (LN) and its efficacy was evaluated in commercial and patient-derived metastatic osteosarcoma cell lines. DOX efficacy was heavily influenced by passage number in metastatic cells, in which an overexpression of P-gp was observed. Notably, DOX-LN overcame the resistance associated with cell passage and improved DOX efficacy fivefold. Moreover, when DOX was co-administered with either free or encapsulated Edelfosine (ET), a synergistic effect was observed. This higher efficacy of the combined treatment was found to be at least partially due to an increase in caspase-dependent cell death. The combination of DOX and ET is thus likely to be effective against osteosarcoma.
Transferrin-conjugated polymeric nanomedicine to enhance the anticancer efficacy of edelfosine in acute myeloid leukemia.[Pubmed:27470549]
Biomed Pharmacother. 2016 Oct;83:51-57.
In this study, transferrin (Tf)-conjugated polyethylene glycol (PEG)-poly-l-lysine (PLL)-poly(lactic-co-glycolic acid) (PLGA) (PEG-PLL-PLGA)-based micellar formulations were successfully prepared for the delivery of Edelfosine (EDS) in leukemia treatment. The micelles were nanosized and presented spherical shaped particles. Our in vitro data suggest that the nanoformulations maintain the biological activity of drugs for longer periods and lead to a continuous release of active drug. The enhanced cellular uptake of EDS-TM resulted in significantly higher cytotoxic effect in K562 leukemia cells. Cell cycle analysis further demonstrated the significantly higher G2/M phase arrest of cancer cells. Immunoblot analysis clearly revealed the potential of EDS-TM in inducing apoptosis of cancer cells which could improve the anticancer efficacy in leukemia. Importantly, EDS-M and EDS-TM significantly prolonged the circulation profile of EDS throughout until 24h, indicating the potential of targeted nanoparticulate delivery system. The prolonged blood circulation potential of micellar formulations might improve the therapeutic potential of drug by increasing its bioavailability in the serum. It would be worthwhile evaluating the effects of the EDS-loaded micelles on cancer cells in vivo for clinical application.
The alkylphospholipid edelfosine shows activity against Strongyloides venezuelensis and induces apoptosis-like cell death.[Pubmed:27394030]
Acta Trop. 2016 Oct;162:180-187.
Strongyloidiasis is widely distributed in the tropical and subtropical areas. Ivermectin is the drug of choice for the treatment. However, the concerns about relying treatment on a single drug make identification of new molecules a priority. Alkylphospholipid analogues, including Edelfosine, are a group of synthetic compounds that have shown activity against some parasites. The objective was to assess the in vitro and in vivo activity of Edelfosine, miltefosine, perifosine against Strongyloides venezuelensis. Moreover, apoptosis-like mechanism in larvae after treatment was studied. Edelfosine displayed the highest activity and the best selectivity index (LD50=49.6 +/- 5.4muM, SI=1.1) compared to miltefosine or perifosine. Third stage larvae after culture with Edelfosine were not able to develop an infection in mice. Treatment of mice with Edelfosine showed reduction of 47% in parasitic females allocated in the gut. Moreover, DNA fragmentation was observed by TUNEL staining in larvae treated with Edelfosine. These results suggest that Edelfosine could be an effective drug against strongyloidiasis, probably through induction of apoptosis-like cell death.
Edelfosine Promotes Apoptosis in Androgen-Deprived Prostate Tumors by Increasing ATF3 and Inhibiting Androgen Receptor Activity.[Pubmed:26944919]
Mol Cancer Ther. 2016 Jun;15(6):1353-63.
Edelfosine is a synthetic alkyl-lysophospholipid that possesses significant antitumor activity in several human tumor models. Here, we investigated the effects of Edelfosine combined with androgen deprivation (AD) in LNCaP and VCaP human prostate cancer cells. This treatment regimen greatly decreased cell proliferation compared with single agent or AD alone, resulting in higher levels of apoptosis in LNCaP compared with VCaP cells. Edelfosine caused a dose-dependent decrease in AKT activity, but did not affect the expression of total AKT in either cell line. Furthermore, Edelfosine treatment inhibited the expression of androgen receptor (AR) and was associated with an increase in activating transcription factor 3 (ATF3) expression levels, a stress response gene and a negative regulator of AR transactivation. ATF3 binds to AR after Edelfosine + AD and represses the transcriptional activation of AR as demonstrated by PSA promoter studies. Knockdown of ATF3 using siRNA-ATF3 reversed the inhibition of PSA promoter activity, suggesting that the growth inhibition effect of Edelfosine was ATF3 dependent. Moreover, expression of AR variant 7 (ARv7) and TMPRSS2-ERG fusion gene were greatly inhibited after combined treatment with AD and Edelfosine in VCaP cells. In vivo experiments using an orthotopic LNCaP model confirmed the antitumor effects of Edelfosine + AD over the individual treatments. A significant decrease in tumor volume and PSA levels was observed when Edelfosine and AD were combined, compared with Edelfosine alone. Edelfosine shows promise in combination with AD for the treatment of prostate cancer patients. Mol Cancer Ther; 15(6); 1353-63. (c)2016 AACR.
Roles of brain phosphatidylinositol-specific phospholipase C and diacylglycerol lipase in centrally administered histamine-induced adrenomedullary outflow in rats.[Pubmed:17628524]
Eur J Pharmacol. 2007 Oct 1;571(2-3):138-44.
Recently, we reported that intracerebroventricularly (i.c.v.) administered histamine evokes the secretion of noradrenaline and adrenaline from adrenal medulla by brain cyclooxygenase-1- and thromboxane A2-mediated mechanisms in rats. These results suggest the involvement of brain arachidonic acid cascade in the histamine-induced activation of the central adrenomedullary outflow. Arachidonic acid is released mainly by phospholipase A2 (PLA2)-dependent pathway or phospholipase C (PLC)/diacylglycerol lipase-dependent pathway. In the present study, histamine (27 nmol/animal, i.c.v.) -induced elevation of plasma noradrenaline and adrenaline was dose-dependently reduced by U-73122 (PLC inhibitor) (10 and 100 nmol/animal, i.c.v.), ET-18-OCH3 (phosphatidylinositol-specific PLC inhibitor) (10 and 30 nmol/animal, i.c.v.) and RHC-80267 (diacylglycerol lipase inhibitor) (1.3 and 2.6 micromol/animal, i.c.v.). However, mepacrine (PLA2 inhibitor) (1.1 and 2.2 micromol/animal, i.c.v.) and D609 (phosphatidylcholine-specific PLC inhibitor) (30, 100 and 300 nmol/animal, i.c.v.) had no effect. These results suggest the involvement of brain phosphatidylinositol-specific PLC and diacylglycerol lipase in the centrally administered histamine-induced activation of the adrenomedullary outflow in rats.
Phospholipase C and myosin light chain kinase inhibition define a common step in actin regulation during cytokinesis.[Pubmed:17509155]
BMC Cell Biol. 2007 May 17;8:15.
BACKGROUND: Phosphatidylinositol 4,5-bisphosphate (PIP2) is required for successful completion of cytokinesis. In addition, both PIP2 and phosphoinositide-specific phospholipase C (PLC) have been localized to the cleavage furrow of dividing mammalian cells. PLC hydrolyzes PIP2 to yield diacylglycerol (DAG) and inositol trisphosphate (IP3), which in turn induces calcium (Ca2+) release from the ER. Several studies suggest PIP2 must be hydrolyzed continuously for continued cleavage furrow ingression. The majority of these studies employ the N-substituted maleimide U73122 as an inhibitor of PLC. However, the specificity of U73122 is unclear, as its active group closely resembles the non-specific alkylating agent N-ethylmaleimide (NEM). In addition, the pathway by which PIP2 regulates cytokinesis remains to be elucidated. RESULTS: Here we compared the effects of U73122 and the structurally unrelated PLC inhibitor ET-18-OCH3 (Edelfosine) on cytokinesis in crane-fly and Drosophila spermatocytes. Our data show that the effects of U73122 are indeed via PLC because U73122 and ET-18-OCH3 produced similar effects on cell morphology and actin cytoskeleton organization that were distinct from those caused by NEM. Furthermore, treatment with the myosin light chain kinase (MLCK) inhibitor ML-7 caused cleavage furrow regression and loss of both F-actin and phosphorylated myosin regulatory light chain from the contractile ring in a manner similar to treatment with U73122 and ET-18-OCH3. CONCLUSION: We have used multiple inhibitors to examine the roles of PLC and MLCK, a predicted downstream target of PLC regulation, in cytokinesis. Our results are consistent with a model in which PIP2 hydrolysis acts via Ca2+ to activate myosin via MLCK and thereby control actin dynamics during constriction of the contractile ring.
A radioreceptor binding assay for platelet-activating factor (PAF) using membranes from CHO cells expressing human PAF receptor.[Pubmed:7594622]
J Immunol Methods. 1995 Oct 26;186(2):225-31.
A simple and reproducible radioreceptor assay (RRA) has been developed using membranes from CHO cells which can stably express human platelet-activating factor (PAF) receptor. The CHO cells expressing the PAF receptor, termed CHO.1F8, showed a significant intracellular Ca2+ response to PAF, and the same binding properties to [3H]WEB 2086, a PAF antagonist, as reported (Kd, 13.6 +/- 1.9 nM; Bmax, 2.5 +/- 0.4 pmol/mg protein (n = 6)). A competitive binding assay was done using the CHO.1F8 cell membranes and [3H]WEB 2086. The minimum detectable dose of PAF was 0.3 nM (approximately 30 pg per well) and the assay was highly specific for PAF. This method makes it possible to handle large numbers of samples rapidly and simultaneously, since the receptor membrane is prepared in advance and the binding assay can be completed within 3 h. Using this method, we have determined the production and cell association of PAF in human neutrophils.
Selective inhibition of phosphatidylinositol phospholipase C by cytotoxic ether lipid analogues.[Pubmed:1316230]
Cancer Res. 1992 May 15;52(10):2835-40.
The ether lipid analogue 1-octadecyl-2-methyl-rac-glycero-3-phosphocholine (ET-18-OCH3) has been shown to be a direct inhibitor of Swiss 3T3 fibroblast and BG1 ovarian adenocarcinoma cell cytosolic phosphoinositide selective phospholipase C (PIPLC) using [3H]-phosphatidylinositol-(4, 5)-bisphosphate ([3H]PIP2) as the substrate. The inhibition occurred when ET-18-OCH3 was incorporated into the [3H]PIP2 substrate micelles, with 50% inhibition (IC50) occurring at a ET-18-OCH3: [3H]PIP2 ratio of 0.04, or an assay concentration of 0.4 microM, and when ET-18-OCH3 was added directly to the incubation, with an IC50 of 9.6 microM. Lipid prepared from cells exposed to cytotoxic concentrations of ET-18-OCH3 for 18 h also inhibited PIPLC with an IC50 less than 1 microM. The noncytotoxic analogue 1-O-alkyl-2-hydroxy-sn-glycero-3-phosphocholine inhibited PIPLC when incorporated into the [3H]PIP2 substrate micelles, but lipid from cells grown with 5 microM 1-O-alkyl-2-hydroxy-sn-glycero-3-phosphocholine did not inhibit PIPLC. BG1 cells, which were more sensitive than Swiss 3T3 fibroblasts to growth inhibition by ET-18-OCH3, had a cytosolic PIPLC activity one-third that of Swiss 3T3 cells. NIH 3T3 cells exhibited the same sensitivity to growth inhibition by ET-18-OCH3 as Swiss 3T3 cells and had a similar level of PIPLC. v-sis NIH 3T3 cells were relatively resistant (greater than 3-fold) to growth inhibition by ET-18-OCH3 and had a cytosolic PIPLC activity more than twice that of the wild type cells. ET-18-OCH3 was a weak inhibitor, IC50 greater than 100 microM, of phospholipase D activity in NIH 3T3 cell membranes. In intact NIH 3T3 cells ET-18-OCH3 at cytotoxic concentrations did not inhibit phospholipase D or phosphatidylcholine-selective phospholipase C activity. The results show that the ether lipid analogues at cytotoxic concentrations are selective inhibitors of PIPLC and that the inhibition of PIPLC may be related to the growth inhibitory activity of the ether lipid analogues.


