(E)-N-CaffeoylputrescineCAS# 29554-26-5 |
Quality Control & MSDS
Number of papers citing our products

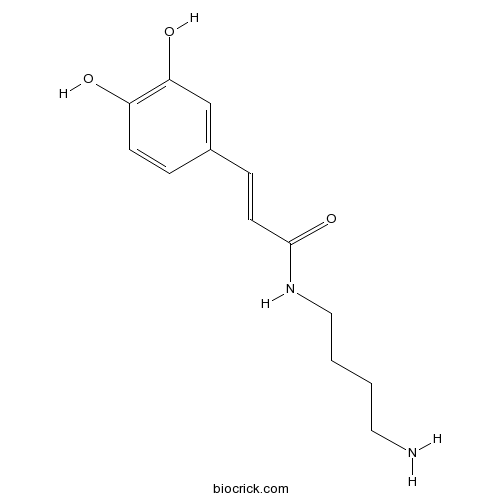
Chemical structure

3D structure
| Cas No. | 29554-26-5 | SDF | Download SDF |
| PubChem ID | 439877 | Appearance | Powder |
| Formula | C13H18N2O3 | M.Wt | 250.3 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | N-(4-aminobutyl)-3-(3,4-dihydroxyphenyl)prop-2-enamide | ||
| SMILES | C1=CC(=C(C=C1C=CC(=O)NCCCCN)O)O | ||
| Standard InChIKey | KTZNZCYTXQYEHT-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C13H18N2O3/c14-7-1-2-8-15-13(18)6-4-10-3-5-11(16)12(17)9-10/h3-6,9,16-17H,1-2,7-8,14H2,(H,15,18) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | N-Caffeoylputrescine has antioxidant activity. |
| In vitro | Antioxidant activity of phenolics in leaves of three red pepper (Capsicum annuum) cultivars.[Pubmed: 24087837 ]J Agric Food Chem. 2014 Jan 29;62(4):850-9.The antioxidant properties and phenolic profiles were first investigated in this paper on the leaves of three red pepper cultivars, Blackcuban (BCPL), Hongjinju (HPL), and Yeokgang-hongjanggun (YHPL). |
| Structure Identification | Phytochemistry (Oxford), 1996, 42(2):389-396.Changes in the accumulation of soluble and cell wall-bound phenolics in elicitor-treated cell suspension cultures and fungus-infected leaves of Solanum tuberosum.[Reference: WebLink]Cell suspension cultures of potato (Solanum tuberosum cv. Datura) treated with an elicitor preparation from Phytophthora infestans and potato leaves infected with the same fungus were used to study changes in the accumulation patterns of soluble and cell wall-bound phenolics.
|

(E)-N-Caffeoylputrescine Dilution Calculator

(E)-N-Caffeoylputrescine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.9952 mL | 19.976 mL | 39.9521 mL | 79.9041 mL | 99.8801 mL |
| 5 mM | 0.799 mL | 3.9952 mL | 7.9904 mL | 15.9808 mL | 19.976 mL |
| 10 mM | 0.3995 mL | 1.9976 mL | 3.9952 mL | 7.9904 mL | 9.988 mL |
| 50 mM | 0.0799 mL | 0.3995 mL | 0.799 mL | 1.5981 mL | 1.9976 mL |
| 100 mM | 0.04 mL | 0.1998 mL | 0.3995 mL | 0.799 mL | 0.9988 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Negletein
Catalog No.:BCN8085
CAS No.:29550-13-8
- Olivil
Catalog No.:BCN5198
CAS No.:2955-23-9
- Eupatoletin
Catalog No.:BCN3605
CAS No.:29536-44-5
- MNI-caged-L-glutamate
Catalog No.:BCC7086
CAS No.:295325-62-1
- Valerosidate
Catalog No.:BCN6750
CAS No.:29505-31-5
- Cypellocarpin C
Catalog No.:BCN7556
CAS No.:294856-66-9
- Narciclasine
Catalog No.:BCN4732
CAS No.:29477-83-6
- Ganoderic acid Z
Catalog No.:BCN2440
CAS No.:294674-09-2
- L 006235
Catalog No.:BCC2361
CAS No.:294623-49-7
- Ticarcillin sodium
Catalog No.:BCC4737
CAS No.:29457-07-6
- Sophorabioside
Catalog No.:BCN7838
CAS No.:2945-88-2
- Pseudolycorine
Catalog No.:BCN5371
CAS No.:29429-03-6
- Sakuranetin
Catalog No.:BCN5199
CAS No.:2957-21-3
- 2-Amino-2',5-dichlorobenzophenone
Catalog No.:BCC8520
CAS No.:2958-36-3
- Gynuramide II
Catalog No.:BCN5200
CAS No.:295803-03-1
- Friedelin 3,4-lactone
Catalog No.:BCN6449
CAS No.:29621-75-8
- 13-Oxo-9E,11E-octadecadienoic acid
Catalog No.:BCN8173
CAS No.:29623-29-8
- 2-Aminothiazol-4-acetic acid
Catalog No.:BCC8556
CAS No.:29676-71-9
- Ethynodiol diacetate
Catalog No.:BCC4483
CAS No.:297-76-7
- Oxyresveratrol
Catalog No.:BCN5201
CAS No.:29700-22-9
- dihydrokaempferol
Catalog No.:BCC8191
CAS No.:5150-32-3
- DOB hydrochloride
Catalog No.:BCC5947
CAS No.:29705-96-2
- Methyl chlorogenate
Catalog No.:BCC9042
CAS No.:29708-87-0
- Clopidol
Catalog No.:BCC8918
CAS No.:2971-90-6


