DihydroevocarpineCAS# 15266-35-0 |
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 15266-35-0 | SDF | Download SDF |
| PubChem ID | 5322031 | Appearance | Powder |
| Formula | C23H35NO | M.Wt | 341.5 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
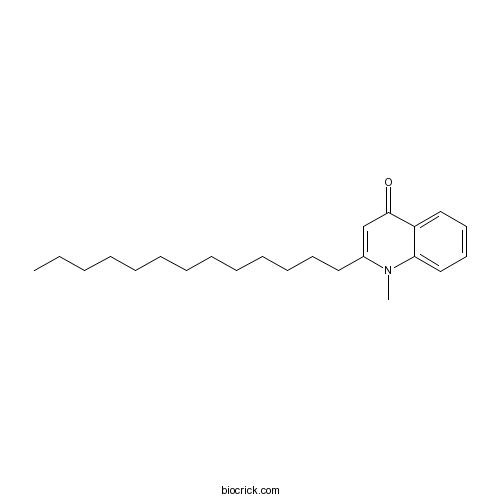
| Chemical Name | 1-methyl-2-tridecylquinolin-4-one | ||
| SMILES | CCCCCCCCCCCCCC1=CC(=O)C2=CC=CC=C2N1C | ||
| Standard InChIKey | DWHCRAGHDDLXEM-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C23H35NO/c1-3-4-5-6-7-8-9-10-11-12-13-16-20-19-23(25)21-17-14-15-18-22(21)24(20)2/h14-15,17-19H,3-13,16H2,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Dihydroevocarpine and 1-methyl-2-undecyl-4-quinolone are moderate modulators of p-glycoprotein (p-gp) activity. 2. Dihydroevocarpine shows more potent inhibitory effects against MAO-B compared to MAO-A. 3. Dihydroevocarpine shows potent anti-Helicobacter pylori activity with the minimum inhibitory concentration (MIC) value of 10-20 microg/ml. |
| Targets | P-gp | Antifection | MAO |

Dihydroevocarpine Dilution Calculator

Dihydroevocarpine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.9283 mL | 14.6413 mL | 29.2826 mL | 58.5652 mL | 73.2064 mL |
| 5 mM | 0.5857 mL | 2.9283 mL | 5.8565 mL | 11.713 mL | 14.6413 mL |
| 10 mM | 0.2928 mL | 1.4641 mL | 2.9283 mL | 5.8565 mL | 7.3206 mL |
| 50 mM | 0.0586 mL | 0.2928 mL | 0.5857 mL | 1.1713 mL | 1.4641 mL |
| 100 mM | 0.0293 mL | 0.1464 mL | 0.2928 mL | 0.5857 mL | 0.7321 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 4-Methyl-2-propyl-1H-benzimidazole-6-carboxylic acid
Catalog No.:BCC8713
CAS No.:152628-03-0
- 2-n-Propyl-4-methyl-6-(1-methylbenzimidazole-2-yl)benzimidazole
Catalog No.:BCC8584
CAS No.:152628-02-9
- Testosterone isocaproate
Catalog No.:BCC9170
CAS No.:15262-86-9
- H-Thr(Bzl)-OBzl.oxalate
Catalog No.:BCC3105
CAS No.:15260-11-4
- Boc-Thr(Bzl)-OH
Catalog No.:BCC3451
CAS No.:15260-10-3
- Buddlejasaponin IVb
Catalog No.:BCN2842
CAS No.:152580-79-5
- BMY 45778
Catalog No.:BCC7068
CAS No.:152575-66-1
- Fmoc-N-Me-Asp(OtBu)-OH
Catalog No.:BCC3212
CAS No.:152548-66-8
- PSB 1115
Catalog No.:BCC7237
CAS No.:152529-79-8
- Nebivolol
Catalog No.:BCC4332
CAS No.:152520-56-4
- Gnetin J
Catalog No.:BCN3384
CAS No.:152511-23-4
- Leachianone G
Catalog No.:BCN3308
CAS No.:152464-78-3
- Evocarpine
Catalog No.:BCN7064
CAS No.:15266-38-3
- Cimiside B
Catalog No.:BCN1679
CAS No.:152685-91-1
- Lyconnotine
Catalog No.:BCN1277
CAS No.:6900-93-2
- PF-06447475
Catalog No.:BCC5589
CAS No.:1527473-33-1
- A 366
Catalog No.:BCC5624
CAS No.:1527503-11-2
- Sevelamer HCl
Catalog No.:BCC4718
CAS No.:152751-57-0
- Puerol A
Catalog No.:BCN6566
CAS No.:152784-32-2
- RS 25344 hydrochloride
Catalog No.:BCC7645
CAS No.:152815-28-6
- Chartarlactam A
Catalog No.:BCN7110
CAS No.:1528745-88-1
- Ginkgolide A
Catalog No.:BCN1680
CAS No.:15291-75-5
- Ginkgolide C
Catalog No.:BCN1681
CAS No.:15291-76-6
- Ginkgolide B
Catalog No.:BCN1682
CAS No.:15291-77-7
[Simultaneous determination of seven constituents in Euodiae Fructus and two related species by HPLC].[Pubmed:25272498]
Zhongguo Zhong Yao Za Zhi. 2014 Jul;39(14):2693-8.
This study is to develop a HPLC method for quality evaluation of Euodiae Fructus and related species by simultaneous determination limonin, indole alkaloids (14-fomyldihydroxyrutaecarpine, evodiamine, rutaecarpine), and quinolone alkaloids [1-methyl-2-undecyl-4 (1H)-quinolone, evocarpine, Dihydroevocarpine] in the fruits of five Evodia species. Samples were analyzed on a YMC C18 column (4.6 mm x 250 mm, 5 microm) eluted with mobile phases of acetonitrile (A), tetrahydrofuran (B), and a buffer solution of 5 mmol x L(-1) ammonium acetate (pH 3.8) (C) in a linear gradient mode. The column temperature was 30 degrees C and the flow rate was 1.0 mL x min(-1). The PDA detector wavelengths were set at 220 and 250 nm. The seven compounds were well separated and showed good linearity (r = 0.999 9) within the concentration ranges tested. The mean recoveries were between 96.7%-102.4% (RSD 1.4%-3.1%). Through the validation, the method was proved to be accurate and repeatable. All the seven constituents were detected in the fruits of five species, but the contents of them varied widely in different samples. The total contents of seven constituents in 16 batches of Euodiae Fructus were 9.46-69.9 mg x g(-1), and the mean content was 28.2 mg x g(-1). The total content of seven constituents in E. compacta and E. fargesii was 25.8, 7.69 mg x g(-1), respectively.
Quinolone alkaloids from evodiae fructus and their inhibitory effects on monoamine oxidase.[Pubmed:17489352]
Arch Pharm Res. 2007 Apr;30(4):397-401.
1-Methyl-2-undecyl-4(1H)-quinolone (1) was previously isolated as a selective MAO-B inhibitor from the Evodiae Fructus. Further bioassay-guided purification led to the identification of five known quinolone alkaloids, 1-methyl-2-nonyl-4(1H)-quinolone (2), 1-methyl-2-[(Z)-6-undecenyl]-4(1H)-quinolone (3), evocarpine (4), 1-methyl-2-[(6Z,9Z)-6,9-pentadecadienyl]-4(1H)-quinolone (5), and Dihydroevocarpine (6). All the isolates showed more potent inhibitory effects against MAO-B compared to MAO-A. The most MAO-B selective compound 5 among the isolates inhibited MAO-B in a competitive manner, according to kinetic analyses by Lineweaver-Burk reciprocal plots.
Anti-Helicobacter pylori activity of quinolone alkaloids from Evodiae fructus.[Pubmed:10549874]
Biol Pharm Bull. 1999 Oct;22(10):1141-3.
A biologically monitored fractionation of methanol extract of the fruit of Evodia rutaecarpa led to the isolation of six quinolone alkaloids, evocarpine (1), 1-methyl-2-[(4Z,7Z)-4,7-tridecadienyl]-4(1H)-quinolone (2), 1-methyl-2-[(6Z,9Z)-6,9-pentadecadienyl]-4(1H)-quinolo ne (3), 1-methyl-2-undecyl-4(1H)-quinolone (4), Dihydroevocarpine (5), 1-methyl-2-pentadecyl-4(1H)-quinolone (6). They showed potent anti-Helicobacter pylori activity with the minimum inhibitory concentration (MIC) value of 10-20 microg/ml. However, they had no effect on Helicobacter pylori urease activity at the concentration of 300 microg/ml.
Cytotoxicity and p-glycoprotein modulating effects of quinolones and indoloquinazolines from the Chinese herb Evodia rutaecarpa.[Pubmed:18058680]
Planta Med. 2007 Dec;73(15):1554-7.
The antimycobacterial quinolones 1-methyl-2-undecyl-4-quinolone, Dihydroevocarpine and evocarpine as well as the indoloquinazoline alkaloids rutaecarpine and evodiamine - all from the Chinese medicinal herb Evodia rutaecarpa - were tested in two in vitro assays, for cytotoxicity and interaction with p-glycoprotein (p-gp). Cytotoxicity was measured in a cell proliferation assay against CCRF-CEM leukemia cells and their p-gp over-expressing subline CEM/ADR5000. An assay monitoring the p-gp-dependent accumulation of the dye calcein in porcine brain capillary endothelial cells (PBCECs) was used to study interactions of the test substances with this efflux pump. Rutaecarpine and evodiamine showed quite high toxicity with IC (50) values from 2.64 to 4.53 microM and were weak modulators of p-gp activity. The degrees of resistance in CEM/ADR5000 towards the saturated quinolones 1-methyl-2-undecyl-4-quinolone and Dihydroevocarpine were between 3 and 4. In the calcein assay, these two quinolones were shown to be moderate modulators of p-gp activity. Evocarpine, on the other side, is not transported by p-gp, and showed only slight toxicity at the highest test concentration of 30 microM.


