DhurrinCAS# 499-20-7 |
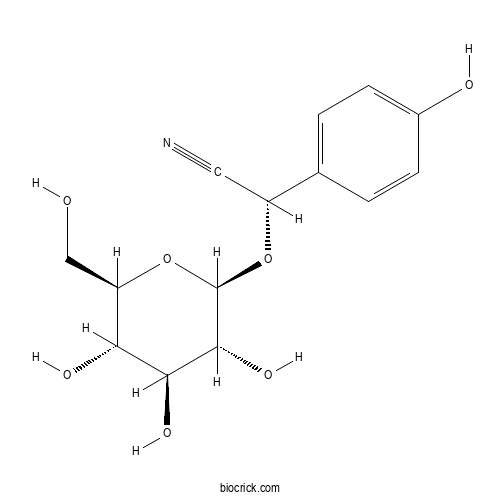
- Taxiphyllin
Catalog No.:BCN4922
CAS No.:21401-21-8
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 499-20-7 | SDF | Download SDF |
| PubChem ID | 161355 | Appearance | Powder |
| Formula | C14H17NO7 | M.Wt | 311.3 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2S)-2-(4-hydroxyphenyl)-2-[(2R,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyacetonitrile | ||
| SMILES | C1=CC(=CC=C1C(C#N)OC2C(C(C(C(O2)CO)O)O)O)O | ||
| Standard InChIKey | NVLTYOJHPBMILU-YOVYLDAJSA-N | ||
| Standard InChI | InChI=1S/C14H17NO7/c15-5-9(7-1-3-8(17)4-2-7)21-14-13(20)12(19)11(18)10(6-16)22-14/h1-4,9-14,16-20H,6H2/t9-,10-,11-,12+,13-,14-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Reference standards. | |||||

Dhurrin Dilution Calculator

Dhurrin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.2123 mL | 16.0617 mL | 32.1234 mL | 64.2467 mL | 80.3084 mL |
| 5 mM | 0.6425 mL | 3.2123 mL | 6.4247 mL | 12.8493 mL | 16.0617 mL |
| 10 mM | 0.3212 mL | 1.6062 mL | 3.2123 mL | 6.4247 mL | 8.0308 mL |
| 50 mM | 0.0642 mL | 0.3212 mL | 0.6425 mL | 1.2849 mL | 1.6062 mL |
| 100 mM | 0.0321 mL | 0.1606 mL | 0.3212 mL | 0.6425 mL | 0.8031 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- (-)-Hydroxycitric acid lactone
Catalog No.:BCN0043
CAS No.:27750-13-6
- (+)-D-3-Carene
Catalog No.:BCN0042
CAS No.:498-15-7
- Hydroxyvalerenic acid
Catalog No.:BCN0041
CAS No.:1619-16-5
- Cucurbitin chloride
Catalog No.:BCN0040
CAS No.:80546-88-9
- Myrcene
Catalog No.:BCN0039
CAS No.:123-35-3
- 1-Octadecanol
Catalog No.:BCN0038
CAS No.:112-92-5
- Steviol 19-glucoside
Catalog No.:BCN0037
CAS No.:64977-89-5
- 8-Acetyl-7-hydroxycoumarin
Catalog No.:BCN0036
CAS No.:6748-68-1
- (1R)-(-)-Menthyl acetate
Catalog No.:BCN0035
CAS No.:2623-23-6
- 4'-Methoxyflavone
Catalog No.:BCN0034
CAS No.:4143-74-2
- Methyl nonadecanoate
Catalog No.:BCN0033
CAS No.:1731-94-8
- Sutherlandioside B
Catalog No.:BCN0032
CAS No.:1055329-47-9
- Cimiracemoside F
Catalog No.:BCN0045
CAS No.:264875-61-8
- Englerin A
Catalog No.:BCN0046
CAS No.:1094250-15-3
- 1-Hexanol
Catalog No.:BCN0047
CAS No.:111-27-3
- Chrysantellin A
Catalog No.:BCN0048
CAS No.:73039-13-1
- (S)-(-)-beta-Citronellol
Catalog No.:BCN0049
CAS No.:7540-51-4
- Gossypetin 3,3',8-trimethylether
Catalog No.:BCN0050
CAS No.:14965-08-3
- N1,N5,N10-(E)-tri-p-coumaroylspermidine
Catalog No.:BCN0051
CAS No.:364368-18-3
- Miliacin
Catalog No.:BCN0052
CAS No.:5945-45-9
- (S)-(-)-3,7-Dimethyl-6-octenal
Catalog No.:BCN0053
CAS No.:5949-05-3
- 6-Hydroxyflavanone
Catalog No.:BCN0054
CAS No.:4250-77-5
- 2-Nonyl alcohol
Catalog No.:BCN0055
CAS No.:628-99-9
- 3-Ethoxy-4-hydroxybenzaldehyde
Catalog No.:BCN0056
CAS No.:121-32-4
Phytochemical constituents and anti-tyrosinase activity of Macadamia integrifolia leaves extract.[Pubmed:33207965]
Nat Prod Res. 2020 Nov 19:1-6.
Macadamia integrifolia Maiden & Betche is cultivated around the world for its highly valued nuts (macadamia nuts). Although the chemical composition of the edible macadamia oil has been repeatedly investigated, other plant organs have not been phytochemically or biologically assessed. In this study, ethanolic extract of M. integrifolia leaves was phytochemically investigated which led to the isolation of 6 compounds. Two functional galactolipids, i.e., monogalactosyl diacylglycrol 36:4 (MGDG 36:4), digalactosyl monoacylglycerol 18:2 (DGMG 18:2), gallic acid and protocatechuic acid were identified in the genus Macadamia for the first time, in addition to the cyanogenic glycoside Dhurrin and beta-sitosterol. Additionally, anti-tyrosinase activity of the extract, its fractions and isolated compounds was investigated and a good tyrosinase inhibitory activity was observed for the extract, IC50=85 microg/mL and its polar fractions (ethyl acetate at 60 microg/mL and n-butanol at 75 microg/mL), with gallic acid showing strong anti-tyrosinase activity at IC50 56 microg/mL.
Prediction of Dhurrin Metabolism by Transcriptome and Metabolome Analyses in Sorghum.[Pubmed:33086681]
Plants (Basel). 2020 Oct 19;9(10). pii: plants9101390.
Sorghum (Sorghum bicolor (L.)) Moench is an important food for humans and feed for livestock. Sorghum contains Dhurrin which can be degraded into toxic hydrogen cyanide. Here, we report the expression patterns of 14 candidate genes related to Dhurrin ((S)-4-Hydroxymandelnitrile-beta-D-glucopyranoside) metabolism and the effects of the gene expression on specific metabolite content in selected sorghum accessions. Dhurrin-related metabolism is vigorous in the early stages of development of sorghum. The Dhurrin contents of most accessions tested were in the range of approximately 6-22 mug mg(-1) fresh leaf tissue throughout growth. The p-hydroxybenzaldehyde (pHB) contents were high at seedling stages, but almost nonexistent at adult stages. The contents of p-hydroxyphenylacetic acid (pHPAAc) were relatively low throughout growth compared to those of Dhurrin or pHB. Generally, the expression of the candidate genes was higher at seedling stage than at other stages and decreased gradually as plants grew. In addition, we identified significant SNPs, and six of them were potentially associated with non-synonymous changes in CAS1. Our results may provide the basis for choosing breeding materials to regulate cyanide contents in sorghum varieties to prevent HCN toxicity of livestock or to promote drought tolerance or pathogen resistance.
Development and validation of eight cyanogenic glucosides via ultra-high-performance liquid chromatography-tandem mass spectrometry in agri-food.[Pubmed:32593038]
Food Chem. 2020 Nov 30;331:127305.
An ultra-high-performance liquid chromatography-triple quadrupole tandem mass spectrometry (UHPLC-QqQ-MS/MS) method was established and validated for the simultaneous quantification of eight cyanogenic glucosides (CNGs) in agri-food. The eight CNGs were linamarin, lotaustralin, linustatin, neolinustatin, taxiphyllin, amygdalin, Dhurrin and prunasin. CNGs were extracted with aqueous methanol and cleaned via solid-phase extraction. Analytes were separated with a C18 column via gradient elution. MS/MS analysis was performed with electrospray ionisation in positive mode. Quantification was performed in multiple reaction monitoring mode. Satisfactory validation results were obtained in terms of linearity, sensitivity, precision and accuracy, matrix effect and stability. The method was applied in typical cyanogenic agri-food. CNGs in cassava, linseed, bamboo, sorghum, apricot, almond and lima bean were analyzed.
Matrix-Assisted Laser Desorption/Ionization-Mass Spectrometry Imaging of Metabolites during Sorghum Germination.[Pubmed:32350122]
Plant Physiol. 2020 Jul;183(3):925-942.
Dhurrin is the most abundant cyanogenic glucoside found in sorghum (Sorghum bicolor) where it plays a key role in chemical defense by releasing toxic hydrogen cyanide upon tissue disruption. Besides this well-established function, there is strong evidence that Dhurrin plays additional roles, e.g. as a transport and storage form of nitrogen, released via endogenous recycling pathways. However, knowledge about how, when and why Dhurrin is endogenously metabolized is limited. We combined targeted metabolite profiling with matrix-assisted laser desorption/ionization-mass spectrometry imaging to investigate accumulation of Dhurrin, its recycling products and key general metabolites in four different sorghum lines during 72 h of grain imbibition, germination and early seedling development, as well as the spatial distribution of these metabolites in two of the lines. Little or no Dhurrin or recycling products were present in the dry grain, but their de novo biosynthesis started immediately after water uptake. Dhurrin accumulation increased rapidly within the first 24 h in parallel with an increase in free amino acids, a key event in seed germination. The trajectories and final concentrations of Dhurrin, the recycling products and free amino acids reached within the experimental period were dependent on genotype. Matrix-assisted laser desorption/ionization-mass spectrometry imaging demonstrated that Dhurrin primarily accumulated in the germinating embryo, confirming its function in protecting the emerging tissue against herbivory. The Dhurrin recycling products, however, were mainly located in the scutellum and/or pericarp/seed coat region, suggesting unknown key functions in germination.
Theoretical Analysis for the Safe Form and Dosage of Amygdalin Product.[Pubmed:32167430]
Anticancer Agents Med Chem. 2020;20(7):897-908.
Indroduction: This article presents a theoretical analysis of the safe form and dosage of the amygdalin derivative. By making a precise socio-anthropological analysis of the life of the ancient people of Botra (Hunza people, Burusho/Brusho people), a hypothesis has been postulated through a number of modern quantum-mechanical, molecular-topological and bio analytical checks, and has also been confirmed by two proofs. METHODS: The proposed hypothesis underwent theoretical and logical analysis to confirm and/or reject it. The methodological scheme was: determining the optimal chemical formula, determination of the pharmaceutical molecular form and determination of the drug dose. RESULTS: A convenient, harmless, form of amygdalin derivative is available that has the same biological and chemical activity and could be used in conservative clinical oncology. The article also presents a theoretical comparative analysis of biochemical reactivity in in vivo and in vitro media, by which we also determine the recommended dosage for patient administration. A comparative analysis of the data, obtained in published clinical studies of amygdalin, is presented, summarizing a scheme of the anti-tumor activity of the proposed molecular form. CONCLUSION: The hydrolyzed to amide / carboxylic acid cyano / nitrile glycosides are potential drugs. Their biological activity remains unchanged, but their toxicity is many times lower than unmodified native molecules. We claim that this study we have conducted on amygdalin / Dhurrin-derived amide is the only study on this molecular form. Other substances in these groups with pronounced biological activity (including anti-tumor) are the hydrolyzed nitrile groups by Prunasin, Lucumin, Vicianin, Sambunigrin, Dhurrin, Taxiphyllin, Zierin, Preteacin, p-Glucosyloxymandelonitrile, Linamarin, Lotaustralin, Acaciapetalin, Triglochinin, Dejdaclin, Tetraphyllin A, Tetrallin B, Gynocardin etc., to their amide/carboxylic acid.
Unique and highly specific cyanogenic glycoside localization in stigmatic cells and pollen in the genus Lomatia (Proteaceae).[Pubmed:32157299]
Ann Bot. 2020 Aug 13;126(3):387-400.
BACKGROUND AND AIMS: Floral chemical defence strategies remain understudied despite the significance of flowers to plant fitness, and the fact that many flowers contain secondary metabolites that confer resistance to herbivores. Optimal defence and apparency theories predict that the most apparent plant parts and/or those most important to fitness should be most defended. To test whether within-flower distributions of chemical defence are consistent with these theories we used cyanogenic glycosides (CNglycs), which are constitutive defence metabolites that deter herbivores by releasing hydrogen cyanide upon hydrolysis. METHODS: We used cyanogenic florets of the genus Lomatia to investigate at what scale there may be strategic allocation of CNglycs in flowers, what their localization reveals about function, and whether levels of floral CNglycs differ between eight congeneric species across a climatic gradient. Within-flower distributions of CNglycs during development were quantified, CNglycs were identified and their localization was visualized in cryosectioned florets using matrix-assisted laser desorption ionization mass spectrometry imaging (MALDI-MSI). KEY RESULTS: Florets of all congeneric species studied were cyanogenic, and concentrations differed between species. Within florets there was substantial variation in CNglyc concentrations, with extremely high concentrations (up to 14.6 mg CN g-1 d. wt) in pollen and loose, specialized surface cells on the pollen presenter, among the highest concentrations reported in plant tissues. Two tyrosine-derived CNglycs, the monoglycoside Dhurrin and diglycoside proteacin, were identified. MALDI-MSI revealed their varying ratios in different floral tissues; proteacin was primarily localized to anthers and ovules, and Dhurrin to specialized cells on the pollen presenter. The mix of transient specialized cells and pollen of L. fraxinifolia was ~11 % Dhurrin and ~1.1 % proteacin by mass. CONCLUSIONS: Tissue-specific distributions of two CNglycs and substantial variation in their concentrations within florets suggests their allocation is under strong selection. Localized, high CNglyc concentrations in transient cells challenge the predictions of defence theories, and highlight the importance of fine-scale metabolite visualization, and the need for further investigation into the ecological and metabolic roles of CNglycs in floral tissues.
Cyanogenesis in Macadamia and Direct Analysis of Hydrogen Cyanide in Macadamia Flowers, Leaves, Husks, and Nuts Using Selected Ion Flow Tube-Mass Spectrometry.[Pubmed:32053983]
Foods. 2020 Feb 11;9(2). pii: foods9020174.
Macadamia has increasing commercial importance in the food, cosmetics, and pharmaceutical industries. However, the toxic compound hydrogen cyanide (HCN) released from the hydrolysis of cyanogenic compounds in Macadamia causes a safety risk. In this study, optimum conditions for the maximum release of HCN from Macadamia were evaluated. Direct headspace analysis of HCN above Macadamia plant parts (flower, leaves, nuts, and husks) was carried out using selected ion flow tube-mass spectrometry (SIFT-MS). The cyanogenic glycoside Dhurrin and total cyanide in the extracts were analyzed using HPLC-MS and UV-vis spectrophotometer, respectively. HCN released in the headspace was at a maximum when Macadamia samples were treated with pH 7 buffer solution and heated at 50 degrees C for 60 min. Correspondingly, treatment of Macadamia samples under these conditions resulted in 93%-100% removal of Dhurrin and 81%-91% removal of total cyanide in the sample extracts. Hydrolysis of cyanogenic glucosides followed a first-order reaction with respect to HCN production where cyanogenesis is principally induced by pH changes initiating enzymatic hydrolysis rather than thermally induced reactions. The effective processing of different Macadamia plant parts is important and beneficial for the safe production and utilization of Macadamia-based products.
The Interplay Between Water Limitation, Dhurrin, and Nitrate in the Low-Cyanogenic Sorghum Mutant adult cyanide deficient class 1.[Pubmed:31798611]
Front Plant Sci. 2019 Nov 15;10:1458.
Sorghum bicolor (L.) Moench produces the nitrogen-containing natural product Dhurrin that provides chemical defense against herbivores and pathogens via the release of toxic hydrogen cyanide gas. Drought can increase Dhurrin in shoot tissues to concentrations toxic to livestock. As Dhurrin is also a remobilizable store of reduced nitrogen and plays a role in stress mitigation, reductions in Dhurrin may come at a cost to plant growth and stress tolerance. Here, we investigated the response to an extended period of water limitation in a unique EMS-mutant adult cyanide deficient class 1 (acdc1) that has a low Dhurrin content in the leaves of mature plants. A mutant sibling line was included to assess the impact of unknown background mutations. Plants were grown under three watering regimes using a gravimetric platform, with growth parameters and Dhurrin and nitrate concentrations assessed over four successive harvests. Tissue type was an important determinant of Dhurrin and nitrate concentrations, with the response to water limitation differing between above and below ground tissues. Water limitation increased Dhurrin concentration in the acdc1 shoots to the same extent as in wild-type plants and no growth advantage or disadvantage between the lines was observed. Lower Dhurrin concentrations in the acdc1 leaf tissue when fully watered correlated with an increase in nitrate content in the shoot and roots of the mutant. In targeted breeding efforts to down-regulate Dhurrin concentration, parallel effects on the level of stored nitrates should be considered in all vegetative tissues of this important forage crop to avoid potential toxic effects.
Stabilization of dhurrin biosynthetic enzymes from Sorghum bicolor using a natural deep eutectic solvent.[Pubmed:31794881]
Phytochemistry. 2020 Feb;170:112214.
In recent years, ionic liquids and deep eutectic solvents (DESs) have gained increasing attention due to their ability to extract and solubilize metabolites and biopolymers in quantities far beyond their solubility in oil and water. The hypothesis that naturally occurring metabolites are able to form a natural deep eutectic solvent (NADES), thereby constituting a third intracellular phase in addition to the aqueous and lipid phases, has prompted researchers to study the role of NADES in living systems. As an excellent solvent for specialized metabolites, formation of NADES in response to dehydration of plant cells could provide an appropriate environment for the functional storage of enzymes during drought. Using the enzymes catalyzing the biosynthesis of the defense compound Dhurrin as an experimental model system, we demonstrate that enzymes involved in this pathway exhibit increased stability in NADES compared with aqueous buffer solutions, and that enzyme activity is restored upon rehydration. Inspired by nature, application of NADES provides a biotechnological approach for long-term storage of entire biosynthetic pathways including membrane-anchored enzymes.
Modulation of Hematological Indices of Normal and Alloxan-Induced Diabetic Rabbits by Aqueous Extract of Pleurotus tuberregium Sclerotia.[Pubmed:31544706]
Endocr Metab Immune Disord Drug Targets. 2020;20(3):380-387.
OBJECTIVE: The ability of an aqueous extract of the sclerotia of Pleurotus tuberregium to modulate hematological parameters was investigated in normal and alloxan treated rabbits. METHODS: The extract was subjected to atomic absorption spectrophotometric and flame ionization detector-coupled-gas chromatographic (GC-FID) analysis. Diabetes mellitus was induced by a 120 mg/kg body weight intravenous injection of alloxan. Metformin was orally administered at 50 mg/kg, while the extract was administered (both to normal and diabetic rabbits) at 100, 200 and 300 mg/kg. RESULTS: Analysis of the extract showed that it had high contents of calcium, magnesium, manganese and potassium. Eleven known glycosides were detected, comprising mainly of amygdalin (37.7%), digoxin (14.4%), Dhurrin (14.0%), linamarin (13.6%), prunasin (10.8%) and digitoxin (8.4%). Also detected were twelve known saponins, consisting mainly of sapogenin (40.3%) and neochlorogenin (21.8%); and twelve known lignans, consisting mainly of matairesinol (59.7%), secoisolariciresinol (20.9%) and lariciresinol (14.9%). Compared to the Diabetic control, the hematocrit, hemoglobin concentration, mean cell hemoglobin, mean cell hemoglobin concentration, mean corpuscular volume, red cell distribution width; and red cell, total white cell, lymphocytes, granulocytes and platelet counts of the treated groups were significantly (p<0.05) higher. CONCLUSION: The above result showed that the extract had a positive effect on the hemopoietic system of the treated animals, at least at the doses at which it was administered in this study.
Down-Regulation of CYP79A1 Gene Through Antisense Approach Reduced the Cyanogenic Glycoside Dhurrin in [Sorghum bicolor (L.) Moench] to Improve Fodder Quality.[Pubmed:31544105]
Front Nutr. 2019 Aug 30;6:122.
A major limitation for the utilization of sorghum forage is the production of the cyanogenic glycoside Dhurrin in its leaves and stem that may cause the death of cattle feeding on it at the pre-flowering stage. Therefore, we attempted to develop transgenic sorghum plants with reduced levels of hydrogen cyanide (HCN) by antisense mediated down-regulation of the expression of cytochrome P450 CYP79A1, the key enzyme of the Dhurrin biosynthesis pathway. CYP79A1 cDNA was isolated and cloned in antisense orientation, driven by rice Act1 promoter. Shoot meristem explants of sorghum cultivar CSV 15 were transformed by the particle bombardment method and 27 transgenics showing the integration of transgene were developed. The biochemical assay for HCN in the transgenic sorghum plants confirmed significantly reduced HCN levels in transgenic plants and their progenies. The HCN content in the transgenics varied from 5.1 to 149.8 mug/g compared to 192.08 mug/g in the non-transformed control on dry weight basis. Progenies with reduced HCN content were advanced after each generation till T3. In T3 generation, progenies of two promising events were tested which produced highly reduced levels of HCN (mean of 62.9 and 76.2 mug/g, against the control mean of 221.4 mug/g). The reduction in the HCN levels of transgenics confirmed the usefulness of this approach for reducing HCN levels in forage sorghum plants. The study effectively demonstrated that the antisense CYP79A1 gene deployment was effective in producing sorghum plants with lower HCN content which are safer for cattle to feed on.
Production of the cyanogenic glycoside dhurrin in yeast.[Pubmed:31110942]
Metab Eng Commun. 2019 May 1;9:e00092.
Cyanogenic glycosides are defense compounds found in a wide range of plant species, including many crops. We demonstrate that the cyanogenic glucoside Dhurrin, naturally found in sorghum, can be produced at high titers in Saccharomyces cerevisiae, constituting the first report of cyanogenic glycoside production in a microbe. Genetic modifications to increase the supply of the Dhurrin precursor tyrosine enabled Dhurrin production in excess of 80mg/L. The Dhurrin-producing yeast strain was used as a chassis to investigate previously uncharacterized enzymes identified close to the biosynthetic gene cluster containing the Dhurrin pathway enzymes. This work shows the potential of heterologous expression in yeast to facilitate investigations of plant cyanogenic glycoside pathways.
Transcriptome Profiling and Genome-Wide Association Studies Reveal GSTs and Other Defense Genes Involved in Multiple Signaling Pathways Induced by Herbicide Safener in Grain Sorghum.[Pubmed:30906302]
Front Plant Sci. 2019 Mar 8;10:192.
Herbicide safeners protect cereal crops from herbicide injury by inducing genes and proteins involved in detoxification reactions, such as glutathione S-transferases (GSTs) and cytochrome P450s (P450s). Only a few studies have characterized gene or protein expression profiles for investigating plant responses to safener treatment in cereal crops, and most transcriptome analyses in response to safener treatments have been conducted in dicot model species that are not protected by safener from herbicide injury. In this study, three different approaches were utilized in grain sorghum (Sorghum bicolor (L.) Moench) to investigate mechanisms involved in safener-regulated signaling pathways. An initial transcriptome analysis was performed to examine global gene expression in etiolated shoot tissues of hybrid grain sorghum following treatment with the sorghum safener, fluxofenim. Most upregulated transcripts encoded detoxification enzymes, including P450s, GSTs, and UDP-dependent glucosyltransferases (UGTs). Interestingly, several of these upregulated transcripts are similar to genes involved with the biosynthesis and recycling/catabolism of Dhurrin, an important chemical defense compound, in these seedling tissues. Secondly, 761 diverse sorghum inbred lines were evaluated in a genome-wide association study (GWAS) to determine key molecular-genetic factors governing safener-mediated signaling mechanisms and/or herbicide detoxification. GWAS revealed a significant single nucleotide polymorphism (SNP) associated with safener-induced response on chromosome 9, located within a phi-class SbGST gene and about 15-kb from a different phi-class SbGST. Lastly, the expression of these two candidate SbGSTs was quantified in etiolated shoot tissues of sorghum inbred BTx623 in response to fluxofenim treatment. SbGSTF1 and SbGSTF2 transcripts increased within 12-hr after fluxofenim treatment but the level of safener-induced expression differed between the two genes. In addition to identifying specific GSTs potentially involved in the safener-mediated detoxification pathway, this research elucidates a new direction for studying both constitutive and inducible mechanisms for chemical defense in cereal crop seedlings.
Molecular snapshots of dynamic membrane-bound metabolons.[Pubmed:30784399]
Methods Enzymol. 2019;617:1-27.
Numerous biosynthetic pathways have been shown to assemble at the surface of cellular membranes into efficient dynamic supramolecular assemblies termed metabolons. In response to environmental stimuli, metabolons assemble on-demand making them highly dynamic and fragile. This transient nature has previously hampered isolation and molecular characterization of dynamic metabolons. In contrast to conventional detergents, which tend to disrupt weak protein-protein interactions and remove lipids, the competence of a styrene maleic acid copolymer to carve out discrete lipid nanodisc from membranes offers immense potential for isolation of intact protein assemblies. Here, we present a method to extract the entire membrane-bound Dhurrin pathway directly from microsomal fractions of the cereal Sorghum bicolor. This detergent-free nanodisc approach may be generally transposed for isolation of entire plant biosynthetic metabolons. This method provides a simple practical toolkit for the study of membrane protein complexes.
Counting the costs: nitrogen partitioning in Sorghum mutants.[Pubmed:32291046]
Funct Plant Biol. 2018 Jun;45(7):705-718.
Long-standing growth/defence theories state that the production of defence compounds come at a direct cost to primary metabolism when resources are limited. However, such trade-offs are inherently difficult to quantify. We compared the growth and nitrogen partitioning in wild type Sorghum bicolor (L.) Moench, which contains the cyanogenic glucoside Dhurrin, with unique mutants that vary in Dhurrin production. The totally cyanide deficient 1 (tcd1) mutants do not synthesise Dhurrin at all whereas mutants from the adult cyanide deficient class 1 (acdc1) have decreasing concentrations as plants age. Sorghum lines were grown at three different concentrations of nitrogen. Growth, chemical analysis, physiological measurements and expression of key genes in biosynthesis and turnover were determined for leaves, stems and roots at four developmental stages. Nitrogen supply, ontogeny, tissue type and genotype were all important determinants of tissue nitrate and Dhurrin concentration and turnover. The higher growth of acdc1 plants strongly supports a growth/defence trade-off. By contrast, tcd1 plants had slower growth early in development, suggesting that Dhurrin synthesis and turnover may be beneficial for early seedling growth rather than being a cost. The relatively small trade-off between nitrate and Dhurrin suggests these may be independently regulated.


