DeoxynivalenolMycotoxin; potent protein synthesis inhibitor CAS# 51481-10-8 |
- Deltarasin hydrochloride
Catalog No.:BCC4270
CAS No.:1440898-82-7
Quality Control & MSDS
Number of papers citing our products

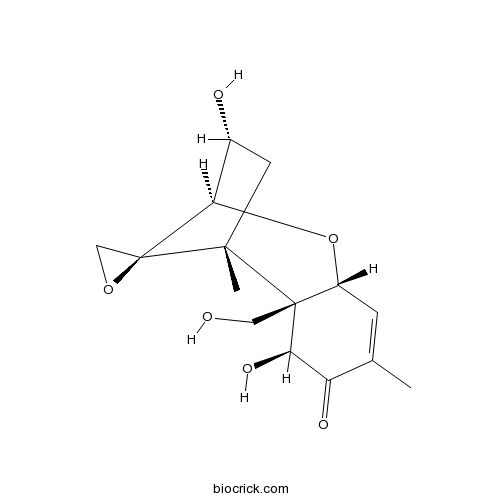
Chemical structure

3D structure
| Cas No. | 51481-10-8 | SDF | Download SDF |
| PubChem ID | 40024 | Appearance | Powder |
| Formula | C15H20O6 | M.Wt | 296.32 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | DON, Vomitoxin | ||
| Solubility | Soluble to 30 mM in ethanol | ||
| SMILES | CC1=CC2C(C(C1=O)O)(C3(CC(C(C34CO4)O2)O)C)CO | ||
| Standard InChIKey | LINOMUASTDIRTM-QGRHZQQGSA-N | ||
| Standard InChI | InChI=1S/C15H20O6/c1-7-3-9-14(5-16,11(19)10(7)18)13(2)4-8(17)12(21-9)15(13)6-20-15/h3,8-9,11-12,16-17,19H,4-6H2,1-2H3/t8-,9-,11-,12-,13-,14-,15+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Tricothecene mycotoxin and potent protein synthesis inhibitor. Exhibits cytotoxic activity in vivo via the ribotoxic stress response. Induces p38-mediated gene expression and apoptosis in leukocytes; activity results in systemic expression of interleukin-6 (IL-6) and other proinflammatory cytokines. Also induces migration of NF-κB into the nucleus. |

Deoxynivalenol Dilution Calculator

Deoxynivalenol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.3747 mL | 16.8737 mL | 33.7473 mL | 67.4946 mL | 84.3683 mL |
| 5 mM | 0.6749 mL | 3.3747 mL | 6.7495 mL | 13.4989 mL | 16.8737 mL |
| 10 mM | 0.3375 mL | 1.6874 mL | 3.3747 mL | 6.7495 mL | 8.4368 mL |
| 50 mM | 0.0675 mL | 0.3375 mL | 0.6749 mL | 1.3499 mL | 1.6874 mL |
| 100 mM | 0.0337 mL | 0.1687 mL | 0.3375 mL | 0.6749 mL | 0.8437 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Cyclo(Tyr-Phe)
Catalog No.:BCN2423
CAS No.:5147-17-1
- COG 133
Catalog No.:BCC1047
CAS No.:514200-66-9
- 3-Methyladenine
Catalog No.:BCC3714
CAS No.:5142-23-4
- Odonicin
Catalog No.:BCN5637
CAS No.:51419-51-3
- Chikusetsusaponin IVa
Catalog No.:BCN3432
CAS No.:51415-02-2
- Alrestatin
Catalog No.:BCC6663
CAS No.:51411-04-2
- Canthaxanthin
Catalog No.:BCC8139
CAS No.:514-78-3
- Biperiden
Catalog No.:BCC4274
CAS No.:514-65-8
- Ferruginol
Catalog No.:BCN3155
CAS No.:514-62-5
- Euphol
Catalog No.:BCN7790
CAS No.:514-47-6
- Tirucallol
Catalog No.:BCN7787
CAS No.:514-46-5
- Parkeol
Catalog No.:BCN3728
CAS No.:514-45-4
- Cimetidine
Catalog No.:BCC4527
CAS No.:51481-61-9
- PMX 205
Catalog No.:BCC8039
CAS No.:514814-49-4
- H-Tyr(tBu)-OMe.HCl
Catalog No.:BCC2672
CAS No.:51482-39-4
- Sclareol
Catalog No.:BCN2395
CAS No.:515-03-7
- (+)-Turicine
Catalog No.:BCC8361
CAS No.:515-24-2
- Cochinchinenin A
Catalog No.:BCN3496
CAS No.:221696-69-1
- Adiantulupanone
Catalog No.:BCN7360
CAS No.:51511-05-8
- GW 803430
Catalog No.:BCC7897
CAS No.:515141-51-2
- Vitexin argininate
Catalog No.:BCC8179
CAS No.:51542-56-4
- Flurizan
Catalog No.:BCC2342
CAS No.:51543-40-9
- Neoechinulin A
Catalog No.:BCN5638
CAS No.:51551-29-2
- DMH4
Catalog No.:BCC6196
CAS No.:515880-75-8
Comparison of Data from a Single-Analyte and a Multianalyte Method for Determination of Urinary Total Deoxynivalenol in Human Samples.[Pubmed:28318271]
J Agric Food Chem. 2017 Aug 23;65(33):7115-7120.
Deoxynivalenol (DON) exposure is estimated by the combined measures of urinary DON and DON-glucuronides. In this study, data from single-mycotoxin (SM) and a multimycotoxin (MM) methods were compared for 256 Swedish adult urine samples. Both methods included beta-glucuronidase predigestion, immunoaffinity enrichment, and LC-MS/MS. However, the specific reagents, apparatus, and conditions were not identical in part because the MM method measures additional mycotoxins. DON was detected in 88 and 63% of samples using the SM and MM methods, respectively, with the following mean and median concentrations: SM, mean = 5.0 ng/mL, SD = 7.4, range of positives = 0.5-60.2 ng/mL, median = 2.5 ng/mL, IQR = 1.0-5.5 ng/mL; MM, mean = 4.4 ng/mL, SD = 12.9, range of positives = 0.5-135.2 ng/mL, median = 0.8 ng/mL, IQR = 0.3-3.5. Linear regression showed a significant, albeit modest, correlation between the two measures (p = 0.0001, r = 0.591). The differences observed may reflect subtle handling differences in DON extraction and quantitation between the methods.
Predicting deoxynivalenol in oats under conditions representing Scandinavian production regions.[Pubmed:28332416]
Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2017 Jun;34(6):1026-1038.
Deoxynivalenol (DON) in cereals, produced by Fusarium fungi, cause poisoning in humans and animals. Fusarium infections in cereals are favoured by humid conditions. Host species are susceptible mainly during the anthesis stage. Infections are also positively correlated with a regional history of Fusarium infections, frequent cereal production and non-tillage field management practices. Here, previously developed process-based models based on relative air humidity, rain and temperature conditions, Fusarium sporulation, host phenology and mycelium growth in host tissue were adapted and tested on oats. Model outputs were used to calculate risk indices. Statistical multivariate models, where independent variables were constructed from weather data, were also developed. Regressions of the risk indices obtained against DON concentrations in field experiments on oats in Sweden and Norway 2012-14 had coefficient of determination values (R(2)) between 0.84 and 0.88. Regressions of the same indices against DON concentrations in oat samples averaged for 11 x 11 km grids in farmers' fields in Sweden 2012-14 resulted in R(2) values between 0.27 and 0.41 for randomly selected grids and between 0.31 and 0.62 for grids with average DON concentration above 1000 mug kg(-)(1) grain in the previous year. When data from all three years were evaluated together, a cross-validated statistical partial least squares model resulted in R(2) = 0.70 and a standard error of cross-validation (SECV) = 522 mug kg(-)(1) grain for the period 1 April-28 August in the construction of independent variables and R(2) = 0.54 and SECV = 647 mug kg(-)(1) grain for 1 April-23 June. Factors that were not accounted for in this study probably explain large parts of the variation in DON among samples and make further model development necessary before these models can be used practically. DON prediction in oats could potentially be improved by combining weather-based risk index outputs with agronomic factors.
Antioxidant activity of JM113 in vitro and its protective effect on broiler chickens challenged with deoxynivalenol.[Pubmed:28380583]
J Anim Sci. 2017 Feb;95(2):837-846.
The aim of this experiment was to study the antioxidant capacity of JM113 isolated from healthy intestinal contents of Tibetan chicken and its protective effect on broiler chickens challenged with Deoxynivalenol (DON). Compared with PZ01 and M23, JM113 demonstrated maximum reducing ( < 0.05) activity and resistance in the presence of 1.2 mmol/L hydrogen peroxide, and great scavenging ability ( < 0.05) against hydroxyl, superoxide anion, and 1,1-diphenyl-2-picrylhydrazyl radicals in vitro. For each strain, the antioxidant activities of live bacterial strains were greater ( < 0.05) than of cell free extracts and dead bacterial strains. To examine the antioxidant capacity of JM113 in vivo, 192 1-d-old Arbor Acres chicks were randomly divided into 4 treatments groups consisting of 6 replicates with 8 birds per replicate. The dietary treatments were 1) control; 2) control diet supplemented with JM113 at 1 x 10 cfu/kg; 3) control diet contaminated with DON at 10 mg/kg; 4) control diet contaminated with DON at 10 mg/kg and supplemented with JM113 at 1 x 10 cfu/kg. Dietary supplementation with DON decreased ( < 0.05) superoxide dismutase activity in serum and increased ( < 0.05) malondialdehyde in the jejunal mucosa of broilers, compared to the control. However, supplementation with JM113 to both the DON-contaminated diet and the control diet, caused a significant reduction ( < 0.05) in malondialdehyde activity in the jejunal mucosa. A reduction ( < 0.05) in expression of nuclear factor erythroid 2-related factor 2 was observed in the jejunal mucosa of broilers fed dietary supplementation with DON, whereas the mRNA levels of and its corresponding downstream gene increased ( < 0.05) with JM113 treatment. Addition of JM113 resulted in longer villi ( < 0.05), even in combination with DON compared to the DON group. JM113 treatment, especially in the DON plus JM113 group, up-regulated ( < 0.05) the expression of mRNA. In conclusion, the present study demonstrates that the JM113 strain has great antioxidant activity and supplementation in feed protected the integrity of the intestinal barrier in broilers challenged with DON, suggesting its use for alleviation of negative effects of DON in poultry.
Draft Genome Sequence of Citrobacter freundii Strain A47, Resistant to the Mycotoxin Deoxynivalenol.[Pubmed:28302773]
Genome Announc. 2017 Mar 16;5(11). pii: 5/11/e00019-17.
Here, we present the draft genome sequence of Citrobacter freundii strain A47 with a length of 4,878,242 bp, which contains 4,357 putative protein coding genes, including 270 unique genes. This work is expected to assist in obtaining novel gene(s) that code for Deoxynivalenol (DON) de-epoxidation enzyme(s).
Lutein protects HT-29 cells against Deoxynivalenol-induced oxidative stress and apoptosis: prevention of NF-kappaB nuclear localization and down regulation of NF-kappaB and Cyclo-Oxygenase-2 expression.[Pubmed:20347963]
Free Radic Biol Med. 2010 Jul 1;49(1):50-60.
Increasing evidence suggests that oxidative stress is closely linked to toxic responses in cells. The tricothecene mycotoxin, Deoxynivalenol (DON), primarily affects cells of the immune system and the GI tract. DON's cytotoxicity is closely linked to intracellular ROS, and it exerts its toxic effect by a mechanism known as ribotoxic stress response, which drives both cytokine expressions at low dosages and apoptosis at high dosages. Studies to alleviate DON's toxicity are sparsely reported in literature. In the present study, the cytoprotective effect of lutein, was tested in HT-29 cells against DON-induced oxidative stress and cytotoxicity. MTT assay revealed IC(20) values of DON at 250 ng/ml. Pre-treatment of cells with 10 microM lutein resulted in 95% cell viability. Lutein combated DON-induced oxidative stress and downregulated expression of inflammatory genes, NF-kappaB and COX-2. Lutein also prevented DON-induced migration of NF-kappaB into the nucleus, as measured by immunofluorescence. Morphological studies by Electron microscopy and Cell cycle analysis by flow cytometry indicated that lutein prevented DON-induced apoptosis. The results of the present study demonstrate for the first time that lutein exerts a cytoprotective role in DON-induced toxicity.
Role of GRP78/BiP degradation and ER stress in deoxynivalenol-induced interleukin-6 upregulation in the macrophage.[Pubmed:19336499]
Toxicol Sci. 2009 Jun;109(2):247-55.
The trichothecene mycotoxin Deoxynivalenol (DON) induces systemic expression of the interleukin-6 (IL-6) and other proinflammatory cytokines in the mouse. The purpose of this study was to test the hypothesis that DON triggers an endoplasmic reticulum (ER) stress response in murine macrophages capable of driving IL-6 gene expression. DON at concentrations up 5000 ng/ml. was not cytotoxic to peritoneal cells. However, DON markedly decreased protein levels but not the mRNA levels of glucose-regulated protein (GRP) 78 (BiP), a chaperone known to mediate ER stress. Inhibitor studies suggested that DON-induced GRP78 degradation was cathepsin and calpain dependent but was proteosome-independent. RNAi-mediated knockdown of GRP78 resulted in increased IL-6 gene expression indicating a potential downregulatory role for this chaperone. GRP78 is critical to the regulation of the two transcription factors, X-box binding protein 1 (XBP1) and activating transcription factor 6 (ATF6), which bind to cAMP-response element (CRE) and drive expression of CRE-dependent genes such as IL-6. DON exposure was found to increase IRE1alpha protein, its modified products spliced XBP1 mRNA and XBP1 protein as well as ATF6. Knockdown of ATF6 but not XBP1 partially inhibited DON-induced IL-6 expression in the macrophages. Three other trichothecenes (satratoxin G, roridin, T-2 toxin) and the ribosome inhibitory protein ricin were also found to induce GRP78 degradation suggesting that other translation inhibitors might evoke ER stress. Taken together, these data suggest that in the macrophage DON induces GRP78 degradation and evokes an ER stress response that could contribute, in part, to DON-induced IL-6 gene expression.
Deoxynivalenol induces p38 interaction with the ribosome in monocytes and macrophages.[Pubmed:18502741]
Toxicol Sci. 2008 Sep;105(1):59-66.
Trichothecene mycotoxins rapidly induce p38-mediated gene expression and apoptosis in mononuclear phagocytes via a process known as the ribotoxic stress response. We hypothesized that the trichothecene Deoxynivalenol (DON) induces interaction of p38 with the ribosome. Two models, U937 human monocytes and RAW 264.7 murine macrophages, were used to test this hypothesis based on their capacity to evoke rapid and robust p38 phosphorylation responses to DON. Following DON treatment of U937 cells, lysates were subjected to sucrose gradient fractionation and the resultant ribosomal fractions probed for p38 by Western blotting. p38 content in fractions containing ribosomal subunits and monosomes (RS + M) increased within 5 min of DON treatment and continued to increase up to 30 min. p38 appeared to be initially interact with the 40S subunit fraction and then subsequently with the 60S unit and monosome fractions. Although p38 phosphorylation was blocked by the inhibitor SB203580, interaction of the kinase with the ribosome was unaffected, suggesting that ribosomal binding and phosphorylation were dissociable events. In RAW 264.7 cells, radiolabeled DON uptake occurred within 15 min and this corresponded to sequential increases nonphosphorylated p38 and phosphorylated p38 in the RS + M fraction. As observed for p38, DON similarly induced both ribosomal interaction with two mitogen-activated protein kinases, c-Jun N-terminal kinase, and extracellular signal-regulated kinase, and their subsequent phosphorylation in RAW 264.7 cells. Taken together, these data suggest that, in mononuclear phagocytes, DON induced p38 mobilization to the ribosome and its subsequent phosphorylation. The ribosome might thus play a central role as a scaffold in the ribotoxic stress response.


