CinnamoylcocaineCAS# 521-67-5 |
Quality Control & MSDS
Number of papers citing our products

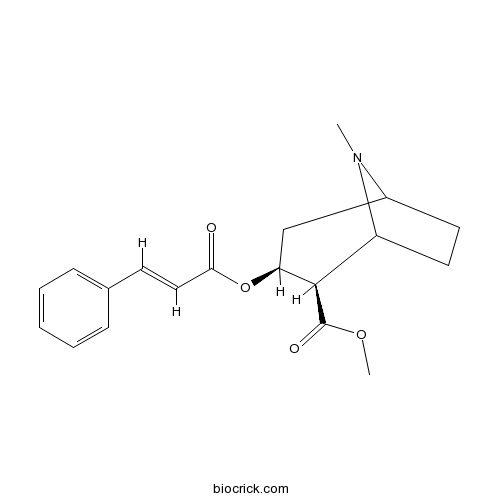
Chemical structure

3D structure
| Cas No. | 521-67-5 | SDF | Download SDF |
| PubChem ID | 5281863 | Appearance | Powder |
| Formula | C19H23NO4 | M.Wt | 329.4 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | methyl (3S,4R)-8-methyl-3-[(E)-3-phenylprop-2-enoyl]oxy-8-azabicyclo[3.2.1]octane-4-carboxylate | ||
| SMILES | CN1C2CCC1C(C(C2)OC(=O)C=CC3=CC=CC=C3)C(=O)OC | ||
| Standard InChIKey | MQIXMJWNEKUAOZ-OSINBPNZSA-N | ||
| Standard InChI | InChI=1S/C19H23NO4/c1-20-14-9-10-15(20)18(19(22)23-2)16(12-14)24-17(21)11-8-13-6-4-3-5-7-13/h3-8,11,14-16,18H,9-10,12H2,1-2H3/b11-8+/t14?,15?,16-,18+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| In vitro | Possibilities for discrimination between chewing of coca leaves and abuse of cocaine by hair analysis including hygrine, cuscohygrine, cinnamoylcocaine and cocaine metabolite/cocaine ratios.[Pubmed: 25138383]Int J Legal Med. 2015 Jan;129(1):69-84.Contrary to the illegal use of any form of manufactured cocaine, chewing of coca leaves and drinking of coca tea are allowed and are very common and socially integrated in several South American countries. Because of this different legal state, an analytical method for discrimination between use of coca leaves and abuse of processed cocaine preparations is required.
|
| Structure Identification | J Forensic Sci. 2007 Jul;52(4):860-6.Four new illicit cocaine impurities from the oxidation of crude cocaine base: formation and characterization of the diastereomeric 2,3-dihydroxy-3-phenylpropionylecgonine methyl esters from cis- and trans-cinnamoylcocaine.[Pubmed: 17553089]
|

Cinnamoylcocaine Dilution Calculator

Cinnamoylcocaine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0358 mL | 15.1791 mL | 30.3582 mL | 60.7165 mL | 75.8956 mL |
| 5 mM | 0.6072 mL | 3.0358 mL | 6.0716 mL | 12.1433 mL | 15.1791 mL |
| 10 mM | 0.3036 mL | 1.5179 mL | 3.0358 mL | 6.0716 mL | 7.5896 mL |
| 50 mM | 0.0607 mL | 0.3036 mL | 0.6072 mL | 1.2143 mL | 1.5179 mL |
| 100 mM | 0.0304 mL | 0.1518 mL | 0.3036 mL | 0.6072 mL | 0.759 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Frangulin A
Catalog No.:BCC8174
CAS No.:521-62-0
- Physcion
Catalog No.:BCN5663
CAS No.:521-61-9
- Vulpic acid
Catalog No.:BCN6546
CAS No.:521-52-8
- Pedicin
Catalog No.:BCN4845
CAS No.:521-51-7
- Cannabinol
Catalog No.:BCN7968
CAS No.:521-35-7
- Sciadopitysin
Catalog No.:BCN5662
CAS No.:521-34-6
- Bilobetin
Catalog No.:BCN5661
CAS No.:521-32-4
- Stanolone
Catalog No.:BCC9153
CAS No.:521-18-6
- Androstenediol
Catalog No.:BCC8828
CAS No.:521-17-5
- Dromostanolone propionate
Catalog No.:BCC8954
CAS No.:521-12-0
- Mestanolone
Catalog No.:BCC9022
CAS No.:521-11-9
- 2-Hydroxy-7-O-methylscillascillin
Catalog No.:BCN5659
CAS No.:52096-50-1
- Broxyquinoline
Catalog No.:BCC4642
CAS No.:521-74-4
- Karanjin
Catalog No.:BCN8370
CAS No.:521-88-0
- 2,4-Dihydroxy-6-methoxy-3-formylacetophenone
Catalog No.:BCN1430
CAS No.:52117-67-6
- 3-O-Acetylpinobanksin
Catalog No.:BCN5660
CAS No.:52117-69-8
- H-Tyr(Bzl)-OBzl.HCl
Catalog No.:BCC3131
CAS No.:52142-01-5
- Piperitol
Catalog No.:BCN3968
CAS No.:52151-92-5
- 7-Hydroxy-2,3,4,5-tetrahydro-1H-benzofuro[2,3-c]azepin-1-one
Catalog No.:BCC3960
CAS No.:521937-07-5
- N'-Methylammodendrine
Catalog No.:BCN2147
CAS No.:52196-10-8
- Evoxine
Catalog No.:BCN5664
CAS No.:522-11-2
- Quercitrin
Catalog No.:BCN5665
CAS No.:522-12-3
- Deguelin
Catalog No.:BCN4804
CAS No.:522-17-8
- Norsanguinarine
Catalog No.:BCN3714
CAS No.:522-30-5
Four new illicit cocaine impurities from the oxidation of crude cocaine base: formation and characterization of the diastereomeric 2,3-dihydroxy-3-phenylpropionylecgonine methyl esters from cis- and trans-cinnamoylcocaine.[Pubmed:17553089]
J Forensic Sci. 2007 Jul;52(4):860-6.
Four new impurities have recently been detected in the gas chromatographic signature profiles of many illicit cocaine hydrochloride exhibits. These impurities are only seen in exhibits that have been oxidized and are most prominent in samples that have been highly oxidized. Exhibits containing these compounds were subjected to gas and liquid chromatographic-mass spectrometric analyses to determine the identity of the impurities. These impurities were subsequently synthesized to verify their structures. Four diastereomeric diols formed from the oxidation of cis- and trans-Cinnamoylcocaine were characterized by nuclear magnetic resonance spectrometry, mass spectrometry, and synthesis. Oxidation of cis-Cinnamoylcocaine in neutral conditions yielded (2R,3R)-dihydroxy-3-phenylpropionylecgonine methyl ester and (2S,3S)-dihydroxy-3-phenylpropionylecgonine methyl ester, while trans-Cinnamoylcocaine produced (2R,3S)-dihydroxy-3-phenylpropionylecgonine methyl ester and (2S,3R)-dihydroxy-3-phenylpropionylecgonine methyl ester. The recent appearance of these new impurities suggests that some illicit cocaine processors have modified their oxidation procedures of crude cocaine base for transformation into illicit refined cocaine hydrochloride.
Possibilities for discrimination between chewing of coca leaves and abuse of cocaine by hair analysis including hygrine, cuscohygrine, cinnamoylcocaine and cocaine metabolite/cocaine ratios.[Pubmed:25138383]
Int J Legal Med. 2015 Jan;129(1):69-84.
Contrary to the illegal use of any form of manufactured cocaine, chewing of coca leaves and drinking of coca tea are allowed and are very common and socially integrated in several South American countries. Because of this different legal state, an analytical method for discrimination between use of coca leaves and abuse of processed cocaine preparations is required. In this study, the applicability of hair analysis for this purpose was examined. Hair samples from 26 Argentinean coca chewers and 22 German cocaine users were analysed for cocaine (COC), norcocaine (NC), benzoylecgonine (BE), ecgonine methyl ester (EME), cocaethylene (CE), Cinnamoylcocaine (CIN), tropacocaine (TRO), cuscohygrine (CUS) and hygrine (HYG) by hydrophilic interaction liquid chromatography (HILIC) in combination with triplequad mass spectrometry (MS/MS) and hybrid quadrupole time-of-flight mass spectrometry (QTOF-MS). The following concentrations (range, median, ng/mg) were determined in hair of the coca chewers: COC 0.085-75.5, 17.0; NC 0.03-1.15, 0.12; BE 0.046-35.5, 6.1; EME 0.014-6.0, 0.66; CE 0.00-13.8, 0.38; CIN 0.005-16.8, 0.79; TRO 0.02-0.16, 0.023; CUS 0.026-26.7, 0.31. In lack of a reference substance, only qualitative data were obtained for HYG, and two metabolites of CUS were detected which were not found in hair of the cocaine users. For interpretation, the concentrations of the metabolites and of the coca alkaloids in relation to cocaine were statistically compared between coca chewers and cocaine users. By analysis of variance (ANOVA) significant differences were found for all analytes (alpha = 0.000 to 0.030) with the exception of TRO (alpha = 0.218). The ratios CUS/COC, CIN/COC and EME/COC appeared to be the most suitable criteria for discrimination between both groups with the means and medians 5-fold to 10-fold higher for coca chewers and a low overlap of the ranges between both groups. The same was qualitatively found for HYG. However, these criteria cannot exclude cocaine use in addition to coca chewing. In this regard screening for typical cutting agents can be helpful and led to the detection of levamisole (21x), lidocaine (6x) and paracetamol (3x) in the 22 samples from German cocaine users, whereas no levamisole, lidocaine (3x) and paracetamol (1x) were found in hair from the Argentinean coca chewers. These criteria have to be confirmed for South American cocaine consumers including smokers of coca paste and may be different because of different composition of the drug and other use habits.


