ChlorothiazideCarbonic anhydrase inhibitor CAS# 58-94-6 |
- Naphthoquine phosphate
Catalog No.:BCC1784
CAS No.:173531-58-3
- Mefloquine hydrochloride
Catalog No.:BCC1737
CAS No.:51773-92-3
- Dihydroartemisinin
Catalog No.:BCN6264
CAS No.:71939-50-9
- SID 26681509
Catalog No.:BCC2362
CAS No.:958772-66-2
Quality Control & MSDS
Number of papers citing our products

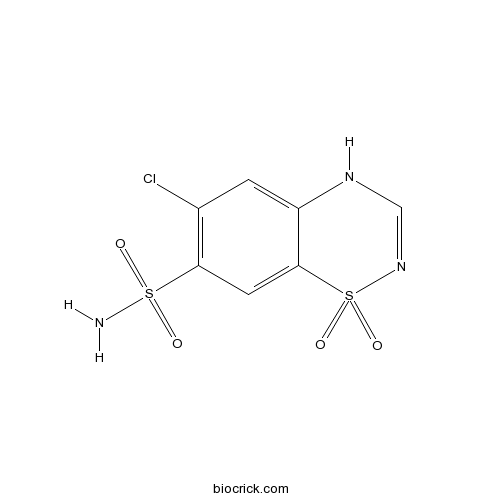
Chemical structure

3D structure
| Cas No. | 58-94-6 | SDF | Download SDF |
| PubChem ID | 2720 | Appearance | Powder |
| Formula | C7H6ClN3O4S2 | M.Wt | 295.72 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 100 mg/mL (338.16 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
| Chemical Name | 6-chloro-1,1-dioxo-4H-1$l^{6},2,4-benzothiadiazine-7-sulfonamide | ||
| SMILES | C1=C2C(=CC(=C1Cl)S(=O)(=O)N)S(=O)(=O)N=CN2 | ||
| Standard InChIKey | JBMKAUGHUNFTOL-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C7H6ClN3O4S2/c8-4-1-5-7(2-6(4)16(9,12)13)17(14,15)11-3-10-5/h1-3H,(H,10,11)(H2,9,12,13) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Chlorothiazide is a diuretic and antihypertensive. (IC50=3.8 mM)
Target: Others
Chlorothiazide sodium (Diuril) is a diuretic used within the hospital setting or for personal use to manage excess fluid associated with congestive heart failure. It is also used as an antihypertensive.
Most often taken in pill form, it is usually taken orally once or twice a day. In the ICU setting, chlorothiazide is given to diurese a patient in addition to furosemide (Lasix). Working in a separate mechanism than furosemide, and absorbed enterically as a reconstituted suspension administered through a nasogastric tube (NG tube), the two drugs potentiate one another. References: | |||||

Chlorothiazide Dilution Calculator

Chlorothiazide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.3816 mL | 16.9079 mL | 33.8158 mL | 67.6315 mL | 84.5394 mL |
| 5 mM | 0.6763 mL | 3.3816 mL | 6.7632 mL | 13.5263 mL | 16.9079 mL |
| 10 mM | 0.3382 mL | 1.6908 mL | 3.3816 mL | 6.7632 mL | 8.4539 mL |
| 50 mM | 0.0676 mL | 0.3382 mL | 0.6763 mL | 1.3526 mL | 1.6908 mL |
| 100 mM | 0.0338 mL | 0.1691 mL | 0.3382 mL | 0.6763 mL | 0.8454 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Chlorothiazide is an inhibitor of carbonic anhydrase and is slightly less potent than acetazolamide. This compound has been shown to block reabsorption of sodium and chloride ions.
- Hydrochlorothiazide
Catalog No.:BCC4786
CAS No.:58-93-5
- D-(+)-Xylose
Catalog No.:BCN1010
CAS No.:58-86-6
- Biotin
Catalog No.:BCC3585
CAS No.:58-85-5
- Papaverine
Catalog No.:BCC8230
CAS No.:58-74-2
- Inosine
Catalog No.:BCN3841
CAS No.:58-63-9
- Adenosine
Catalog No.:BCN5796
CAS No.:58-61-7
- Puromycin aminonucleoside
Catalog No.:BCC1873
CAS No.:58-60-6
- Puromycin dihydrochloride
Catalog No.:BCC7860
CAS No.:58-58-2
- Pyridoxine HCl
Catalog No.:BCC4835
CAS No.:58-56-0
- Theophylline
Catalog No.:BCN1258
CAS No.:58-55-9
- Tetrabenazine
Catalog No.:BCC5277
CAS No.:58-46-8
- Prochlorperazine
Catalog No.:BCC3846
CAS No.:58-38-8
- alpha-Tocopherol acetate
Catalog No.:BCN5803
CAS No.:58-95-7
- Uridine
Catalog No.:BCN4090
CAS No.:58-96-8
- 6-Aminoquinoline
Catalog No.:BCC8766
CAS No.:580-15-4
- 3-Aminoquinoline
Catalog No.:BCC8620
CAS No.:580-17-6
- 2-Aminoquinoline
Catalog No.:BCC8555
CAS No.:580-22-3
- Matairesinol
Catalog No.:BCN5789
CAS No.:580-72-3
- Epicorynoxidine
Catalog No.:BCN7554
CAS No.:58000-48-9
- HOKU-81
Catalog No.:BCC1634
CAS No.:58020-43-2
- Averantin
Catalog No.:BCN7027
CAS No.:5803-62-3
- 24, 25-Dihydroxy VD2
Catalog No.:BCC1302
CAS No.:58050-55-8
- Miltefosine
Catalog No.:BCC4360
CAS No.:58066-85-6
- trans-3,4-Methylenedioxycinnamyl alcohol
Catalog No.:BCN1410
CAS No.:58095-76-4
Intravenous Chlorothiazide Versus Enteral Metolazone to Augment Loop Diuretic Therapy in the Intensive Care Unit.[Pubmed:28228057]
Ann Pharmacother. 2017 Apr;51(4):286-292.
BACKGROUND: In cases of loop diuretic resistance in the intensive care unit (ICU), recommendations for a specific second-line thiazide agent are lacking. OBJECTIVE: To compare the effects of intravenous Chlorothiazide (CTZ) and enteral metolazone (MET) on urine output (UOP) when added to furosemide monotherapy therapy in critically ill adults. METHODS: This was a retrospective cohort study conducted in the medical, surgical, and cardiothoracic ICUs of a quaternary medical center. The primary outcome was change in UOP induced by the study interventions compared with furosemide alone. Secondary outcomes included onset of diuresis, eventual need for hemodialysis, and incidence of adverse events. RESULTS: A total of 122 patients (58 in CTZ, 64 in MET) were included. When added to furosemide monotherapy, CTZ induced a greater change in UOP at 24 hours compared with MET (2405 vs 1646 mL, respectively; P = 0.01). CTZ also caused a more rapid dieresis: 1463 mL total UOP in the first 6 hours compared with 796 mL in the MET group ( P < 0.01). There were no differences found regarding ICU length of stay, need for renal replacement therapy, or survival to discharge. The CTZ arm required more potassium supplementation to maintain normokalemia (median 100 vs 57 mEq in MET; P = 0.02) and carried a higher cost (mean $97 vs $8, P < 0.01). CONCLUSION: Both CTZ and MET induced significant increases in UOP. CTZ induced a greater and more rapid change and was associated with higher cost and greater need for potassium replacement. Randomized controlled trials are needed to establish whether a preferable thiazide diuretic exists in this setting.
Efficacy of sequential nephron blockade with intravenous chlorothiazide to promote diuresis in cardiac intensive care infants.[Pubmed:27834164]
Cardiol Young. 2017 Aug;27(6):1104-1109.
BACKGROUND: Sequential nephron blockade using intravenous Chlorothiazide is often used to enhance urine output in patients with inadequate response to loop diuretics. A few data exist to support this practice in critically ill infants. METHODS: We included 100 consecutive patients <1 year of age who were administered intravenous Chlorothiazide while receiving furosemide therapy in the cardiac ICU in our study. The primary end point was change in urine output 24 hours after Chlorothiazide administration, and patients were considered to be responders if an increase in urine output of 0.5 ml/kg/hour was documented. Data on demographic, clinical, fluid intake/output, and furosemide and Chlorothiazide dosing were collected. Multivariable regression analyses were performed to determine variables significant for increase in urine output after Chlorothiazide administration. RESULTS: The study population was 48% male, with a mean weight of 4.9+/-1.8 kg, and 69% had undergone previous cardiovascular surgery. Intravenous Chlorothiazide was initiated at 89 days (interquartile range 20-127 days) of life at a dose of 4.6+/-2.7 mg/kg/day (maximum 12 mg/kg/day). Baseline estimated creatinine clearance was 83+/-42 ml/minute/1.73 m2. Furosemide dose before Chlorothiazide administration was 2.8+/-1.4 mg/kg/day and 3.3+/-1.5 mg/kg/day after administration. A total of 43% of patients were categorised as responders, and increase in furosemide dose was the only variable significant for increase in urine output on multivariable analysis (p<0.05). No graphical trends were noted for change in urine output and dose of Chlorothiazide. CONCLUSIONS: Sequential nephron blockade with intravenous Chlorothiazide was not consistently associated with improved urine output in critically ill infants.
Efficacy and Safety of Intravenous Chlorothiazide versus Oral Metolazone in Patients with Acute Decompensated Heart Failure and Loop Diuretic Resistance.[Pubmed:27393709]
Pharmacotherapy. 2016 Aug;36(8):852-60.
STUDY OBJECTIVE: To assess the efficacy and safety of intravenous (IV) Chlorothiazide versus oral metolazone when added to loop diuretics in patients with acute decompensated heart failure (ADHF) and loop diuretic resistance. DESIGN: Retrospective cohort study. SETTING: Large urban academic medical center. PATIENTS: Adults admitted with ADHF between 2005 and 2015 who had loop diuretic resistance, defined as administration of IV furosemide at a dose of 160 mg/day or higher (or an equivalent dose of IV bumetanide), during hospitalization, and who then received at least one dose of IV Chlorothiazide (88 patients) or oral metolazone (89 patients) to augment diuresis. MEASUREMENTS AND MAIN RESULTS: The primary efficacy end point was a change in 24-hour net urine output (UOP) from before to after thiazide-type diuretic administration, and the study was designed to test for the noninferiority of metolazone. Safety end points included changes in renal function and electrolyte concentrations. The mean dose of IV loop diuretic therapy (in IV furosemide equivalents) at baseline (before thiazide-type diuretic administration) was higher in the Chlorothiazide group (mean +/- SD 318.9 +/- 127.7 vs 268.4 +/- 97.6 mg/day in the metolazone group, p=0.004), but net UOP was similar (mean +/- SD 877.0 +/- 1189.0 ml in the Chlorothiazide group vs 710.6 +/- 1145.9 ml in the metolazone group, p=0.344). Mean doses of Chlorothiazide and metolazone were 491 +/- 282 mg and 5.8 +/- 3.5 mg, respectively. Following thiazide-type diuretic administration, net UOP improved to a similar degree (2274.6 +/- 1443.0 ml vs 2030.2 +/- 1725.0 ml in the Chlorothiazide and metolazone groups, respectively, p=0.308). For the primary efficacy end point, metolazone met the threshold for noninferiority by producing a net UOP of 1319.6 +/- 1517.4 ml versus 1397.6 +/- 1370.7 ml for Chlorothiazide (p=0.026 for noninferiority). No significant differences in renal function were observed between the groups. Although hypokalemia was more frequent in the Chlorothiazide group (75% with Chlorothiazide vs 60.7% with metolazone, p=0.045), no significant differences in the rates of severe hypokalemia or other electrolyte abnormalities were observed between the groups. CONCLUSION: Oral metolazone was noninferior to IV Chlorothiazide for enhancing net UOP in patients with ADHF and loop diuretic resistance and was similarly safe with regard to renal function and electrolyte abnormalities. Given the significant cost disparity between the two agents, these findings suggest that oral metolazone may be considered a first-line option in this patient population.


