CP 775146CAS# 702680-17-9 |
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 702680-17-9 | SDF | Download SDF |
| PubChem ID | 46911787 | Appearance | Powder |
| Formula | C26H33NO4 | M.Wt | 423.54 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO | ||
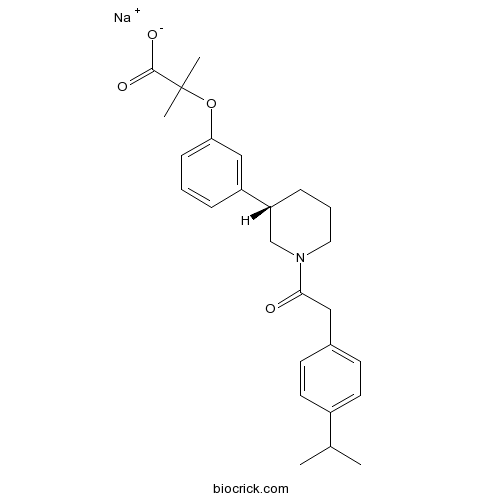
| Chemical Name | sodium;2-methyl-2-[3-[(3S)-1-[2-(4-propan-2-ylphenyl)acetyl]piperidin-3-yl]phenoxy]propanoate | ||
| SMILES | CC(C)C1=CC=C(C=C1)CC(=O)N2CCCC(C2)C3=CC(=CC=C3)OC(C)(C)C(=O)[O-].[Na+] | ||
| Standard InChIKey | YQAGREGJJZRFDN-VZYDHVRKSA-M | ||
| Standard InChI | InChI=1S/C26H33NO4.Na/c1-18(2)20-12-10-19(11-13-20)15-24(28)27-14-6-8-22(17-27)21-7-5-9-23(16-21)31-26(3,4)25(29)30;/h5,7,9-13,16,18,22H,6,8,14-15,17H2,1-4H3,(H,29,30);/q;+1/p-1/t22-;/m1./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective, high affinity PPARα agonist (Ki values are 24.5 nM for PPARα and >10 μM for PPARβ and PPARγ in vitro). Exhibits hypolipidemic activity in vivo. |

CP 775146 Dilution Calculator

CP 775146 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3611 mL | 11.8053 mL | 23.6105 mL | 47.221 mL | 59.0263 mL |
| 5 mM | 0.4722 mL | 2.3611 mL | 4.7221 mL | 9.4442 mL | 11.8053 mL |
| 10 mM | 0.2361 mL | 1.1805 mL | 2.3611 mL | 4.7221 mL | 5.9026 mL |
| 50 mM | 0.0472 mL | 0.2361 mL | 0.4722 mL | 0.9444 mL | 1.1805 mL |
| 100 mM | 0.0236 mL | 0.1181 mL | 0.2361 mL | 0.4722 mL | 0.5903 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- BX795
Catalog No.:BCC3635
CAS No.:702675-74-9
- BX-912
Catalog No.:BCC1250
CAS No.:702674-56-4
- Physalin H
Catalog No.:BCN7917
CAS No.:70241-09-7
- (±)-Lauroylcarnitine chloride
Catalog No.:BCC6690
CAS No.:7023-03-2
- 8alpha-Hydroxy-alpha-gurjunene
Catalog No.:BCN4265
CAS No.:70206-70-1
- 3-Aminoadamantan-1-ol
Catalog No.:BCC8618
CAS No.:702-82-9
- Taranabant
Catalog No.:BCC1985
CAS No.:701977-09-5
- Papain Inhibitor
Catalog No.:BCC1024
CAS No.:70195-20-9
- Isotetrandrine N-2'-oxide
Catalog No.:BCN4264
CAS No.:70191-83-2
- Liquiritic acid
Catalog No.:BCN8332
CAS No.:10379-72-3
- Talopram hydrochloride
Catalog No.:BCC7579
CAS No.:7013-41-4
- DGAT-1 inhibitor
Catalog No.:BCC1529
CAS No.:701232-20-4
- Ivermectin
Catalog No.:BCC1251
CAS No.:70288-86-7
- Fluoroorotic Acid, Ultra Pure
Catalog No.:BCC1209
CAS No.:703-95-7
- Pertussis Toxin
Catalog No.:BCC7565
CAS No.:70323-44-3
- 3-Benzoylthiazolidine-2-thione
Catalog No.:BCC8624
CAS No.:70326-37-3
- Methyl Kakuol
Catalog No.:BCN8243
CAS No.:70342-29-9
- Avobenzone
Catalog No.:BCC4891
CAS No.:70356-09-1
- Sideritoflavone
Catalog No.:BCN6689
CAS No.:70360-12-2
- Lornoxicam
Catalog No.:BCC4425
CAS No.:70374-39-9
- 6alpha-Hydroxynidorellol
Catalog No.:BCN4598
CAS No.:70387-38-1
- Voleneol
Catalog No.:BCN4266
CAS No.:70389-88-7
- 4(15),11-Oppositadien-1-ol
Catalog No.:BCN4267
CAS No.:70389-96-7
- Dihydrotamarixetin
Catalog No.:BCN4268
CAS No.:70411-27-7
Recent perspectives of cerebral palsy (CP) in children: a review.[Pubmed:28353322]
Minerva Pediatr. 2017 Mar 27. pii: S0026-4946.17.04880-0.
The movement and posture disorder of cerebral palsy (CP) is presumed to mainly be a consequence of the motor disorder, but accompanying disturbances with sensations and perception have also been suggested to influence motor function. The heterogeneous condition of cerebral palsy (CP) is caused by an injury to the immature brain affecting movement and posture development. The attainment of standing and walking can be difficult and an assistive device to accomplish the tasks may be required for some children with CP. In this review, we enlightened the role of possible sensory and perceptual disturbances for standing difficulties in children with CP.
Mechanism study of humic acid functional groups for Cr(VI) retention: Two-dimensional FTIR and (13)C CP/MAS NMR correlation spectroscopic analysis.[Pubmed:28355575]
Environ Pollut. 2017 Jun;225:86-92.
Undissolved humic acid (HA) is known to substantially effect the migration and transformation of hexavalent chromium [Cr(VI)] in soils. The mechanisms of Cr(VI) retention in soils by undissolved HA have been reported; however, past studies are inconclusive about the types of HA functional groups that are involved in Cr(VI) retention and the retention mechanisms. Utilizing a two-dimensional correlation spectroscopy (2DCOS) analysis for FTIR and (13)C CP/MAS NMR, this study investigated the variations of HA function groups and molecular structures after reactions with aqueous Cr(VI) under different pH conditions. Based on the changing sequence of functional groups interpreted from the 2DCOS results, a four-step mechanism for Cr(VI) retention was determined as follows: (1) electrostatic adsorption of Cr(VI) to HA surface, (2) complexation of adsorbed Cr(VI) by carboxyl and ester, (3) reduction of complexed Cr(VI) to Cr(III) by phenol and polysaccharide, and (4) complexation of reduced Cr(III) by carboxylic groups. These functional groups that are involved in Cr(VI) retention were determined to occur in aromatic domains.
Cp*Rh(III)-Catalyzed Mild Addition of C(sp(3))-H Bonds to alpha,beta-Unsaturated Aldehydes and Ketones.[Pubmed:28375012]
Org Lett. 2017 Apr 21;19(8):2086-2089.
A Rh(III)-catalyzed addition of benzylic C(sp(3))-H bond to alpha,beta-unsaturated ketones/aldehydes has been realized, leading to efficient synthesis of gamma-aryl ketones/aldehydes. This atom-economic reaction proceeded under mild and redox-neutral conditions with a broad substrate scope. Besides benzylic C-H, allylic C-H bonds are also applicable when assisted by O-methyl ketoxime directing groups.
Molecular characterization of novel and selective peroxisome proliferator-activated receptor alpha agonists with robust hypolipidemic activity in vivo.[Pubmed:18971326]
Mol Pharmacol. 2009 Feb;75(2):296-306.
The nuclear receptor peroxisome proliferator-activated receptor alpha (PPARalpha) is recognized as the primary target of the fibrate class of hypolipidemic drugs and mediates lipid lowering in part by activating a transcriptional cascade that induces genes involved in the catabolism of lipids. We report here the characterization of three novel PPARalpha agonists with therapeutic potential for treating dyslipidemia. These structurally related compounds display potent and selective binding to human PPARalpha and support robust recruitment of coactivator peptides in vitro. These compounds markedly potentiate chimeric transcription systems in cell-based assays and strikingly lower serum triglycerides in vivo. The transcription networks induced by these selective PPARalpha agonists were assessed by transcriptional profiling of mouse liver after short- and long-term treatment. The induction of several known PPARalpha target genes involved with fatty acid metabolism were observed, reflecting the expected pharmacology associated with PPARalpha activation. We also noted the down-regulation of a number of genes related to immune cell function, the acute phase response, and glucose metabolism, suggesting that these compounds may have anti-inflammatory action in the mammalian liver. Whereas these compounds are efficacious in acute preclinical models, extended safety studies and further clinical testing will be required before the full therapeutic promise of a selective PPARalpha agonist is realized.


