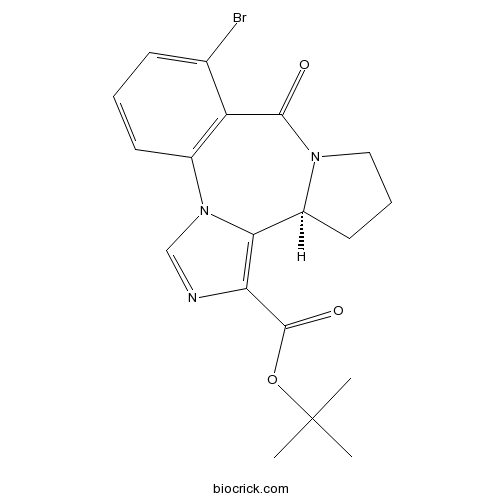
BretazenilBenzodiazepine partial agonist CAS# 84379-13-5 |
- ARL 67156 trisodium salt
Catalog No.:BCC7004
CAS No.:1021868-83-6
- Lansoprazole
Catalog No.:BCC1058
CAS No.:103577-45-3
- Sodium Orthovanadate
Catalog No.:BCC3856
CAS No.:13721-39-6
- BTB06584
Catalog No.:BCC5106
CAS No.:219793-45-0
- Resibufogenin
Catalog No.:BCN5366
CAS No.:465-39-4
- Omeprazole
Catalog No.:BCC1254
CAS No.:73590-58-6
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 84379-13-5 | SDF | Download SDF |
| PubChem ID | 107926 | Appearance | Powder |
| Formula | C19H20BrN3O3 | M.Wt | 418.28 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Ro 16-6028 | ||
| Solubility | Soluble to 100 mM in DMSO and to 100 mM in ethanol | ||
| SMILES | CC(C)(C)OC(=O)C1=C2C3CCCN3C(=O)C4=C(N2C=N1)C=CC=C4Br | ||
| Standard InChIKey | LWUDDYHYYNNIQI-ZDUSSCGKSA-N | ||
| Standard InChI | InChI=1S/C19H20BrN3O3/c1-19(2,3)26-18(25)15-16-13-8-5-9-22(13)17(24)14-11(20)6-4-7-12(14)23(16)10-21-15/h4,6-7,10,13H,5,8-9H2,1-3H3/t13-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Partial agonist at the GABAA benzodiazepine site (EC50 = 10 nM at α1β1γ2 receptors). Displays anticonvulsive activity in vivo. |

Bretazenil Dilution Calculator

Bretazenil Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3907 mL | 11.9537 mL | 23.9074 mL | 47.8149 mL | 59.7686 mL |
| 5 mM | 0.4781 mL | 2.3907 mL | 4.7815 mL | 9.563 mL | 11.9537 mL |
| 10 mM | 0.2391 mL | 1.1954 mL | 2.3907 mL | 4.7815 mL | 5.9769 mL |
| 50 mM | 0.0478 mL | 0.2391 mL | 0.4781 mL | 0.9563 mL | 1.1954 mL |
| 100 mM | 0.0239 mL | 0.1195 mL | 0.2391 mL | 0.4781 mL | 0.5977 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Neuromedin S (rat)
Catalog No.:BCC6055
CAS No.:843782-19-4
- Adoxosidic acid
Catalog No.:BCN7593
CAS No.:84375-46-2
- Mifepristone
Catalog No.:BCC4486
CAS No.:84371-65-3
- Bedaquiline
Catalog No.:BCC5246
CAS No.:843663-66-1
- Rabdosin B
Catalog No.:BCN3236
CAS No.:84304-92-7
- 4,4'-Cyclohexylidenebisphenol
Catalog No.:BCC8663
CAS No.:843-55-0
- Pterosin D 3-O-glucoside
Catalog No.:BCN4567
CAS No.:84299-80-9
- Aliarin
Catalog No.:BCN3919
CAS No.:84294-77-9
- 5-O-Methylvisammioside
Catalog No.:BCN4954
CAS No.:84272-85-5
- Raclopride
Catalog No.:BCC7184
CAS No.:84225-95-6
- AKT Kinase Inhibitor
Catalog No.:BCC1335
CAS No.:842148-40-7
- Canagliflozin
Catalog No.:BCC3696
CAS No.:842133-18-0
- alpha-Arbutin
Catalog No.:BCN8336
CAS No.:84380-01-8
- 23-Hydroxybetulin
Catalog No.:BCN6463
CAS No.:84414-40-4
- Fmoc-NH2
Catalog No.:BCC2803
CAS No.:84418-43-9
- AT7519
Catalog No.:BCC2541
CAS No.:844442-38-2
- A-769662
Catalog No.:BCC2080
CAS No.:844499-71-4
- β-CCB
Catalog No.:BCC6635
CAS No.:84454-35-3
- threo-Guaiacylglycerol-beta-O-4'-dehydrodisinapyl ether
Catalog No.:BCN6928
CAS No.:844637-85-0
- C-1
Catalog No.:BCC6687
CAS No.:84468-24-6
- BTS 54-505 hydrochloride
Catalog No.:BCC5901
CAS No.:84484-78-6
- Sibutramine hydrochloride
Catalog No.:BCC5252
CAS No.:84485-00-7
- Varlitinib (ARRY334543)
Catalog No.:BCC3725
CAS No.:845272-21-1
- Sevelamer Carbonate
Catalog No.:BCC4717
CAS No.:845273-93-0
Self-administration of bretazenil under progressive-ratio schedules: behavioral economic analysis of the role intrinsic efficacy plays in the reinforcing effects of benzodiazepines.[Pubmed:20800977]
Drug Alcohol Depend. 2011 Jan 15;113(2-3):157-64.
Previous research suggests that intrinsic efficacy of benzodiazepines is an important determinant of their behavioral effects. We evaluated the reinforcing effects of the benzodiazepine partial agonist Bretazenil using behavioral economic models referred to as "consumer demand" and "labor supply". Four rhesus monkeys were trained under a progressive-ratio (PR) schedule of i.v. midazolam injection. A range of doses of Bretazenil (0.001-0.03 mg/kg/injection and vehicle) was evaluated for self-administration with an initial response requirement of 40 that doubled to 640; significant self-administration was maintained at doses of 0.003-0.03 mg/kg/injection. Next, a dose of Bretazenil that maintained peak injections/session was made available with initial response requirements doubling from 10 to 320 (maximum possible response requirements of 160 and 5120, respectively), and increasing response requirements decreased self-administration (mean number of injections/session) of a peak dose (0.01 mg/kg/injection). Analyses based on consumer demand revealed that a measure of reinforcing strength termed "essential value", for Bretazenil was similar to that previously obtained with midazolam (non-selective full agonist), but less than that observed for zolpidem (full agonist, selective for alpha1 subunit-containing GABA(A) receptors). According to labor supply analysis, the reinforcing effects of Bretazenil were influenced by the economic concept referred to as a "price effect", similar to our previous findings with midazolam but not zolpidem. In general, behavioral economic indicators of reinforcing effectiveness did not differentiate Bretazenil from a non-selective full agonist. These findings raise the possibility that degree of intrinsic efficacy of a benzodiazepine agonist may not be predictive of relative reinforcing effectiveness.
Efficacy of bretazenil against cortical epileptic afterdischarges increases during early ontogeny in rats.[Pubmed:16963798]
Pharmacol Rep. 2006 Jul-Aug;58(4):519-25.
The effect of a benzodiazepine partial agonist Bretazenil on cortical epileptic afterdischarges was studied in 12-, 18- or 25-day-old rat pups with implanted electrodes. Afterdischarges were induced by low-frequency stimulation of sensorimotor area four times in each animal and Bretazenil (0.1; 1; 5; 10; or 25 mg/kg) and/or solvent were administered intraperitoneally between the first and second stimulation. Bretazenil was able to decrease the duration of afterdischarges as well as intensity of clonic seizures accompanying these afterdischarges in all age groups in a dose-dependent manner. The efficacy of Bretazenil was age-dependent: the most marked effect on duration of afterdischarges was observed in 25-day-old rats. It is in sharp contrast with our earlier data for full agonists clonazepam and midazolam demonstrating the strongest action in the youngest group.
Comparison of the novel subtype-selective GABAA receptor-positive allosteric modulator NS11394 [3'-[5-(1-hydroxy-1-methyl-ethyl)-benzoimidazol-1-yl]-biphenyl-2-carbonitrile] with diazepam, zolpidem, bretazenil, and gaboxadol in rat models of inflammatory and neuropathic pain.[Pubmed:18791060]
J Pharmacol Exp Ther. 2008 Dec;327(3):969-81.
Spinal administration of GABA(A) receptor modulators, such as the benzodiazepine drug diazepam, partially alleviates neuropathic hypersensitivity that manifests as spontaneous pain, allodynia, and hyperalgesia. However, benzodiazepines are hindered by sedative impairments and other side effect issues occurring mainly as a consequence of binding to GABA(A) receptors containing the alpha(1) subunit. Here, we report on the novel subtype-selective GABA(A) receptor-positive modulator NS11394 [3'-[5-(1-hydroxy-1-methyl-ethyl)-benzoimidazol-1-yl]-biphenyl-2-carbonitrile], which possesses a functional efficacy selectivity profile of alpha(5) > alpha(3) > alpha(2) > alpha(1) at GABA(A) alpha subunit-containing receptors. Oral administration of NS11394 (1-30 mg/kg) to rats attenuated spontaneous nociceptive behaviors in response to hindpaw injection of formalin and capsaicin, effects that were blocked by the benzodiazepine site antagonist flumazenil. Ongoing inflammatory nociception, observed as hindpaw weight-bearing deficits after Freund's adjuvant injection, was also completely reversed by NS11394. Likewise, hindpaw mechanical allodynia was fully reversed by NS11394 in two rat models of peripheral neuropathic pain. Importantly, NS11394-mediated antinociception occurred at doses 20 to 40-fold lower than those inducing minor sedative or ataxic impairments. In contrast, putative antinociception associated with administration of either diazepam, zolpidem, or gaboxadol only occurred at doses producing intolerable side effects, whereas Bretazenil was completely inactive despite minor influences on motoric function. In electrophysiological studies, NS11394 selectively attenuated spinal nociceptive reflexes and C-fiber-mediated wind-up in vitro pointing to involvement of a spinal site of action. The robust therapeutic window seen with NS11394 in animals suggests that compounds with this in vitro selectivity profile could have potential benefit in clinical treatment of pain in humans.
Comparative cue generalization profiles of L-838, 417, SL651498, zolpidem, CL218,872, ocinaplon, bretazenil, zopiclone, and various benzodiazepines in chlordiazepoxide and zolpidem drug discrimination.[Pubmed:16339395]
J Pharmacol Exp Ther. 2006 Mar;316(3):1291-9.
The zolpidem discriminative cue is mediated by GABA(A)-alpha1 receptors, whereas the chlordiazepoxide cue may be mediated via non-alpha1 GABA(A) receptors because compounds with selective affinity for GABA(A)-alpha1 receptors fully generalize to the former cue. We predicted that L-838,417 [7-tert-butyl-3-(2,5-difluorophenyl)-6-(2-methyl-2H-1,2,4-triazol-3-ylmethoxy)-1, 2,4-triazolo[4,3-b]pyridazine], a partial agonist at non-alpha1 GABA(A) receptors and an antagonist at GABA(A)-alpha1 receptors, would generalize to the chlordiazepoxide but not the zolpidem-discriminative cue. SL651498 [6-fluoro-9-methyl-2-phenyl-4-(pyrrolidin-1-yl-carbonyl)-2,9-dihydro-1H-pyridol[3 ,4-b]indol-1-one] is a full agonist at GABA(A)-alpha2 receptors, with lower efficacy at GABA(A)-alpha3 receptors and least efficacy at GABA(A)-alpha1 and GABA(A)-alpha5 receptors. Because SL651498 has efficacy at GABA(A)-alpha1 receptors, we anticipated that it would generalize to both discriminative cues. Rats were trained to discriminate either zolpidem (3 mg/kg) or chlordiazepoxide (5 mg/kg) from vehicle using a two-lever operant procedure. The generalization profiles of L-838,417 and SL651498 were compared with nonselective full agonists, GABA(A)-alpha1-selective ligands zolpidem and CL218,872 [3-methyl-6-[3-(trifluoromethyl)phenyl]-1,2,4-triazolo[4,3-b]pyridazine], the nonselective partial agonist Bretazenil, and the novel anxioselective drug ocinaplon. A nonselective partial agonist was included because L-838,417 and SL651498 are partial agonists at some GABA(A) receptors, and this property may influence their generalization profiles. All nonselective full agonists and ocinaplon fully generalized to both cues. CL218,872 and zolpidem generalized to zolpidem only, whereas L-838,417 fully generalized to chlordiazepoxide only. SL651498 fully generalized to chlordiazepoxide and occasioned significant zolpidem-appropriate responding. Bretazenil was similar to SL651498. In conclusion, at this training dose, the chlordiazepoxide-discriminative stimulus is mediated primarily via non-alpha1 GABA(A) receptors and the generalization profiles of the ligands tested seem to correspond with their in vitro profiles at GABA(A) receptor subtypes.
Anticonvulsant tolerance and withdrawal characteristics of benzodiazepine receptor ligands in different seizure models in mice. Comparison of diazepam, bretazenil and abecarnil.[Pubmed:7473156]
J Pharmacol Exp Ther. 1995 Nov;275(2):693-702.
The use of benzodiazepines (BDZs) in the long-term treatment of epilepsy is greatly restricted by their capacity to induce tolerance and dependence. Thus, the development of new BDZ-related therapeutic agents should be directed by strategies that minimize tolerance- and dependence-inducing properties. Experimental procedures used to determine the success of such strategies often rely on a single assay procedure (e.g., one seizure model), which might lead to false predictions. Furthermore, the different types of tolerance, i.e., "pharmacological" (metabolic or functional) and "behavioral" ("learned" or "contingent"), are often not dealt with in such studies. This prompted us to compare the chronic anticonvulsant efficacy and withdrawal characteristics of diazepam and two novel BDZ receptor ligands, i.e., the partial agonist Bretazenil and the subtype-selective agonist abecarnil, in different seizure models in mice. Myoclonic, clonic and tonic seizures were induced by i.v. infusion of pentylenetetrazol and by transcorneal or transauricular application of electrical stimuli. Prolonged administration of diazepam (5 mg/kg twice daily for 6 days) resulted in marked anticonvulsant effects on myoclonic, clonic and tonic seizure thresholds at the onset of treatment, but pronounced tolerance developed rapidly during subsequent treatment. The time course and extent of tolerance was similar with most seizure models. Tolerance characteristics were not affected by study design, i.e., use of separate or the same animals for each seizure induction, indicating that learned or contingent tolerance was not significantly involved under these experimental conditions. After termination of treatment with diazepam, significant seizure threshold decreases were determined, indicating withdrawal hyperexcitability in response to physical dependence. During prolonged administration of abecarnil (10 mg/kg twice daily for 6 days), some anticonvulsant tolerance was seen with electroshock seizures, but not with pentylenetetrazol seizures; no withdrawal hyperexcitability was determined upon termination of treatment. Bretazenil (10 mg/kg twice daily for 6 days) produced no tolerance in any of the seizure models, but a significant decrease in electroshock seizure threshold was seen in the withdrawal period. The data indicate that tolerance and withdrawal characteristics of BDZ receptor partial and subtype-selective agonists in mice depend on the experimental model used, whereas the influence of the experimental protocol is less critical in the case of a full BDZ receptor agonist such as diazepam.


