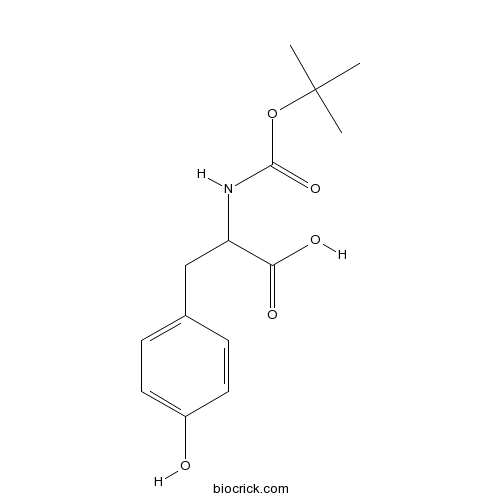
Boc-Tyr-OHCAS# 3978-80-1 |
- Cisplatin
Catalog No.:BCN1552
CAS No.:14283-03-5
- Z-VAD-FMK
Catalog No.:BCC1126
CAS No.:187389-52-2
- Z-WEHD-FMK
Catalog No.:BCC1139
CAS No.:210345-00-9
- Z-LEHD-FMK
Catalog No.:BCC5117
CAS No.:210345-04-3
- AZ 10417808
Catalog No.:BCC2356
CAS No.:331645-84-2
- Apoptosis Activator 2
Catalog No.:BCC2099
CAS No.:79183-19-0
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 3978-80-1 | SDF | Download SDF |
| PubChem ID | 100117 | Appearance | Powder |
| Formula | C14H19NO5 | M.Wt | 281.3 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 3-(4-hydroxyphenyl)-2-[(2-methylpropan-2-yl)oxycarbonylamino]propanoic acid | ||
| SMILES | CC(C)(C)OC(=O)NC(CC1=CC=C(C=C1)O)C(=O)O | ||
| Standard InChIKey | CNBUSIJNWNXLQQ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C14H19NO5/c1-14(2,3)20-13(19)15-11(12(17)18)8-9-4-6-10(16)7-5-9/h4-7,11,16H,8H2,1-3H3,(H,15,19)(H,17,18) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Boc-Tyr-OH Dilution Calculator

Boc-Tyr-OH Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.5549 mL | 17.7746 mL | 35.5492 mL | 71.0985 mL | 88.8731 mL |
| 5 mM | 0.711 mL | 3.5549 mL | 7.1098 mL | 14.2197 mL | 17.7746 mL |
| 10 mM | 0.3555 mL | 1.7775 mL | 3.5549 mL | 7.1098 mL | 8.8873 mL |
| 50 mM | 0.0711 mL | 0.3555 mL | 0.711 mL | 1.422 mL | 1.7775 mL |
| 100 mM | 0.0355 mL | 0.1777 mL | 0.3555 mL | 0.711 mL | 0.8887 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Boc-Tyr-OH
- 2-Amino-4-hydroxy-6-methylpyrimidine
Catalog No.:BCC8531
CAS No.:3977-29-5
- Bryonolol
Catalog No.:BCN2703
CAS No.:39765-50-9
- Dehydrovomifoliol
Catalog No.:BCN7562
CAS No.:39763-33-2
- 16,16-Dimethyl Prostaglandin E2
Catalog No.:BCC7843
CAS No.:39746-25-3
- H-Gln-OtBu.HCl
Catalog No.:BCC2918
CAS No.:39741-62-3
- SDZ 21009
Catalog No.:BCC7098
CAS No.:39731-05-0
- Gue 1654
Catalog No.:BCC6274
CAS No.:397290-30-1
- Daphmacropodine
Catalog No.:BCN5450
CAS No.:39729-21-0
- Catharticin
Catalog No.:BCN6850
CAS No.:39723-40-5
- 2-Benzylsuccinic acid
Catalog No.:BCC8566
CAS No.:3972-36-9
- 1,5-Diphenylpentan-1-one
Catalog No.:BCN7169
CAS No.:39686-51-6
- (R)-Reticuline
Catalog No.:BCN6795
CAS No.:3968-19-2
- Azatadine dimaleate
Catalog No.:BCC4536
CAS No.:3978-86-7
- Penciclovir
Catalog No.:BCC4695
CAS No.:39809-25-1
- Taibaihenryiins A
Catalog No.:BCN3281
CAS No.:398129-59-4
- Epitulipinolide diepoxide
Catalog No.:BCN5451
CAS No.:39815-40-2
- H-Ala-OiPr.HCl
Catalog No.:BCC3193
CAS No.:39825-33-7
- Amikacin disulfate
Catalog No.:BCC4622
CAS No.:39831-55-5
- Methyllinderone
Catalog No.:BCN5452
CAS No.:3984-73-4
- 19-Hydroxybufalin
Catalog No.:BCN8237
CAS No.:39844-86-5
- JNJ 5207852 dihydrochloride
Catalog No.:BCC6101
CAS No.:398473-34-2
- PHA-680632
Catalog No.:BCC2178
CAS No.:398493-79-3
- H-D-Phe(4-OMe)-OH
Catalog No.:BCC2633
CAS No.:39878-65-4
- 29-Hydroxyfriedelan-3-one
Catalog No.:BCN5453
CAS No.:39903-21-4
The synthesis, distribution, and anti-hepatic cancer activity of YSL.[Pubmed:15336278]
Bioorg Med Chem. 2004 Sep 15;12(18):4989-94.
YSL was prepared stepwise from C terminal to N terminal with the side chain un-protective amino acids, Boc-Leu-OMe, Boc-Ser-OH, and Boc-Tyr-OH, as the starting materials in 39.5% total yield (31.2g/per batch). With the side chain un-protective Boc-(3,5-dibromo)-Tyr-OH and HCl.Ser-Leu-OMe as the starting materials (3,5-(3)H-Tyr)-Ser-Leu-OH was obtained in 29% yield. The determination of radioactive quantity in the urine and feces indicated that even after the administration for 130 h only 8.4% (5.35% in urine and 3.05% in feces) of total radioactive quantity from the metabolite of [3,5-(3)H-Tyr]-Ser-Leu-OH were monitored. The distribution study revealed the relative accumulation level of the individual tissue was arranged in the sequence of spleen>liver>kidney>lung>heart>muscle>brain. Selecting hepatic cancer as the target YSL significantly increased the survival time of H22 tumor cells implanted mice.
Amino acids and peptides. LII. Design and synthesis of opioid mimetics containing a pyrazinone ring and examination of their opioid receptor binding activity.[Pubmed:9775433]
Chem Pharm Bull (Tokyo). 1998 Sep;46(9):1374-82.
An amino group was introduced to the 3 or 6 position of a pyrazinone ring by cyclization of dipeptidyl chloromethyl ketones. Boc-Tyr-OH was coupled with the amino function, followed by removal of the Boc group to give pyrazinone ring-containing tyrosine derivatives. Of the various tyrosine derivatives prepared, 5-methyl-6-beta-phenethyl-3-tyrosylaminobutyl-2(1H)-pyrazinone exhibited strong binding to the mu-opioid receptor with a Ki value of 55.8 nM and to the delta-opioid receptor with a Ki value of 2165 nM and with a Ki mu/Ki delta value of 0.026.
An efficient synthesis of N-tert-butoxycarbonyl-O-cyclohexyl-L-tyrosine.[Pubmed:11217115]
Chem Pharm Bull (Tokyo). 2001 Feb;49(2):233-5.
A facile and efficient synthesis of N-tert-butoxycarbonyl-O-cyclohexyl-L-tyrosine [Boc-Tyr(Chx)-OH] is described. Boc-Tyr-OH was treated with NaH in dimethylformamide and then with 3-bromocyclohexene to give N-Boc-O-(cyclohex-2-enyl)-L-tyrosine [Boc-Tyr(Che)-OH] in 70% yield. Hydrogenation of Boc-Tyr(Che)-OH over PtO2 afforded Boc-Tyr(Chx)-OH in almost quantitative yield. The highest yield was achieved when a side product in the synthesis of Boc-Tyr-OH, Boc-Tyr(Boc)-OH, was not removed, because it was also converted to Boc-Tyr(Che)-OH without any additional manipulations. The new synthetic method described here is convenient for practical use, and would facilitate the widespread use of the Chx group for the hydroxy-protection of Tyr.


