Benzyl carbamateCAS# 621-84-1 |
Quality Control & MSDS
Number of papers citing our products

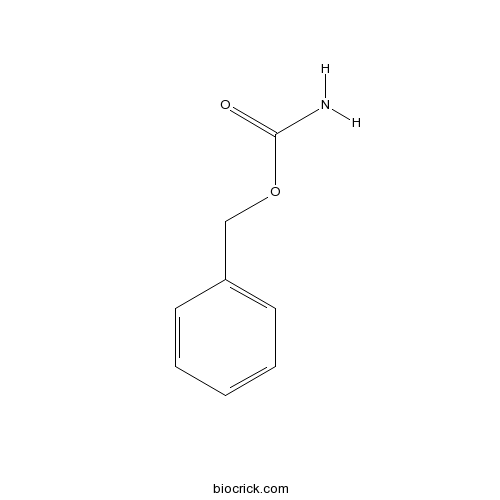
Chemical structure

3D structure
| Cas No. | 621-84-1 | SDF | Download SDF |
| PubChem ID | 12136 | Appearance | Powder |
| Formula | C8H9NO2 | M.Wt | 151 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | benzyl carbamate | ||
| SMILES | C1=CC=C(C=C1)COC(=O)N | ||
| Standard InChIKey | PUJDIJCNWFYVJX-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C8H9NO2/c9-8(10)11-6-7-4-2-1-3-5-7/h1-5H,6H2,(H2,9,10) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Benzyl carbamate Dilution Calculator

Benzyl carbamate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 6.6225 mL | 33.1126 mL | 66.2252 mL | 132.4503 mL | 165.5629 mL |
| 5 mM | 1.3245 mL | 6.6225 mL | 13.245 mL | 26.4901 mL | 33.1126 mL |
| 10 mM | 0.6623 mL | 3.3113 mL | 6.6225 mL | 13.245 mL | 16.5563 mL |
| 50 mM | 0.1325 mL | 0.6623 mL | 1.3245 mL | 2.649 mL | 3.3113 mL |
| 100 mM | 0.0662 mL | 0.3311 mL | 0.6623 mL | 1.3245 mL | 1.6556 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Scutebarbatine E
Catalog No.:BCN8396
CAS No.:910099-77-3
- Isovanillin
Catalog No.:BCN2502
CAS No.:621-59-0
- 3-Nitro-L-tyrosine
Catalog No.:BCN2213
CAS No.:621-44-3
- Heliotrine N-oxide
Catalog No.:BCN1983
CAS No.:6209-65-0
- Nudiposide
Catalog No.:BCN7437
CAS No.:62058-46-2
- Hydroxycitric acid
Catalog No.:BCN2912
CAS No.:6205-14-7
- Onitisin 2'-O-glucoside
Catalog No.:BCN4150
CAS No.:62043-53-2
- 4-(Dimethylamino)cinnamaldehyde
Catalog No.:BCN4968
CAS No.:6203-18-5
- Ginsenoside F3
Catalog No.:BCN1077
CAS No.:62025-50-7
- Ginsenoside F2
Catalog No.:BCN1245
CAS No.:62025-49-4
- Juncusol
Catalog No.:BCN2926
CAS No.:62023-90-9
- Cyclobenzaprine HCl
Catalog No.:BCC6496
CAS No.:6202-23-9
- Rauwolscine hydrochloride
Catalog No.:BCC6834
CAS No.:6211-32-1
- Boc-Phe(4-Br)-OH
Catalog No.:BCC3159
CAS No.:62129-39-9
- Boc-Phe(4-I)-OH
Catalog No.:BCC3260
CAS No.:62129-44-6
- N-Methylcyclohexaneethaneamine
Catalog No.:BCN1795
CAS No.:62141-38-2
- Z-Prolinol
Catalog No.:BCC2709
CAS No.:6216-63-3
- (-)-Syringaresinol
Catalog No.:BCN3417
CAS No.:6216-81-5
- Mirandin B
Catalog No.:BCN6581
CAS No.:62163-24-0
- Benzyl L-(+)-mandelate
Catalog No.:BCC8873
CAS No.:62173-99-3
- 1,2-Bis(phenylthio)ethane
Catalog No.:BCC8415
CAS No.:622-20-8
- Hordenine sulfate
Catalog No.:BCC8184
CAS No.:622-64-0
- 12-O-Methylcarnosic acid
Catalog No.:BCN7655
CAS No.:62201-71-2
- 1,2,3-Triacetyl-5-deoxy-D-ribose
Catalog No.:BCC8408
CAS No.:62211-93-2
Two-step Synthesis of Solasodine Pivalate from Diosgenin Pivalate.[Pubmed:30901960]
Molecules. 2019 Mar 21;24(6). pii: molecules24061132.
A two-step synthesis of solasodine pivalate from diosgenin pivalate is described. The key transformation involves the reaction of diosgenin pivalate with Benzyl carbamate (CbzNH(2)) promoted by TMSOTf. During the reaction the F-ring of the spiroketal moiety opens up with a simultaneous introduction of a Cbz-protected amino group in position 26. A one-pot deprotection of 26-amine with AcBr/BuOH followed by the N-cyclization affords solasodine pivalate in 45% overall yield.
Electrochemical Hydrogenation with Gaseous Ammonia.[Pubmed:30549399]
Angew Chem Int Ed Engl. 2019 Feb 4;58(6):1759-1763.
As a carbon-free and sustainable fuel, ammonia serves as high-energy-density hydrogen-storage material. It is important to develop new reactions able to utilize ammonia as a hydrogen source directly. Herein, we report an electrochemical hydrogenation of alkenes, alkynes, and ketones using ammonia as the hydrogen source and carbon electrodes. A variety of heterocycles and functional groups, including for example sulfide, benzyl, Benzyl carbamate, and allyl carbamate were well tolerated. Fast stepwise electron transfer and proton transfer processes were proposed to account for the transformation.
Dehydrative glycosidations of 2-deoxysugar derivatives catalyzed by an arylboronic ester.[Pubmed:30368103]
Carbohydr Res. 2018 Dec;470:42-49.
An N-methylpyridinium-4-boronic ester acts as a catalyst for dehydrative glycosidations of 2-deoxy sugar-derived hemiacetals. The catalytic protocol is tolerant of functionalized acceptors, including alcohols bearing isopropylidene ketal, tert-butyl carbamate or Benzyl carbamate groups. The results demonstrate that organoboron-catalyzed substitution reactions of alcohols, which have previously been conducted on pi-activated (benzylic, allylic or propargylic) substrates, can also be used to achieve C-O bond formation from carbohydrate-derived hemiacetals.
Synthesis of 2-Azaadamantan-6-one: A Missing Isomer.[Pubmed:30288462]
ACS Omega. 2018 Sep 30;3(9):11362-11367.
2-Azaadamantan-6-one and its Boc and ethylene ketal derivatives were synthesized from 9-oxo endo-bicyclo[3.3.1]non-6-ene-3-carboxylic acid. Similarly, the Cbz, Boc, and ethylene ketal derivatives of 2-azaadamantan-4-one were synthesized from endo-bicyclo[3.3.1]non-6-ene-3-carboxylic acid. Key steps were Curtius rearrangements to form Benzyl carbamates, followed by spontaneous intramolecular attack of the carbamate nitrogen on transient bromonium ion or epoxide intermediates to effect ring closure to azaadamantane intermediates. The reaction sequence leading to 2-azaadamantan-6-one is consistent with the formation of a transient tetracyclic keto aziridine intermediate.
Inhibition of carbonic anhydrases by a substrate analog: benzyl carbamate directly coordinates the catalytic zinc ion mimicking bicarbonate binding.[Pubmed:30140816]
Chem Commun (Camb). 2018 Sep 11;54(73):10312-10315.
N-Unsubstituted carbamates have scarcely been investigated so far as carbonic anhydrase inhibitors (CAIs). By means of kinetic and structural studies, in this paper we demonstrate that such molecules can effectively inhibit hCAs and can be used as lead compounds for the development of CAIs possessing a binding mode similar to one of the CA substrates, bicarbonate.
Development of highly sensitive fluorescent probes for the detection of beta-galactosidase activity - application to the real-time monitoring of senescence in live cells.[Pubmed:29774897]
Analyst. 2018 May 29;143(11):2680-2688.
We report the development of four novel fluorescent probes to monitor the activity of the beta-galactosidase enzyme (beta-gal), in vitro and in living cells. The fluorophores are based on a 6-amino-styryl-benzothiazole push-pull core and display a strong ICT emission. The probes encompass the fluorescent motif that is connected to a beta-d-galactopyranoside moiety through a self-immolative Benzyl carbamate linker (betaGal-1-4). The screening of four different fluorophores enabled us to access new light-up and two-band ratiometric reporters. The four probes, betaGal-1-4, exhibited an extremely fast response and over 200-fold fluorescence enhancement (betaGal-1) following the enzymatic cleavage of the beta-d-galactopyranoside unit. This rapid and extremely sensitive response allowed the detection of senescence-associated beta-galactosidase (SA-beta-gal) activity; a widely used biomarker of senescence. More importantly, betaGal-1 also enabled us to monitor, in real-time, the emergence of senescence in live cells, i.e. the phenotypic transformation from normal to senescent cell. These findings underpin the fact that betaGal-1 may find useful applications in biomedical research. Importantly, betaGal-1 is suitable for epifluorescence and confocal microscopies, and flow cytometry techniques, which are among the most common analytical tools in biology.


