BMS-564929Androgen receptor (AR) modulator CAS# 627530-84-1 |
- AZD3514
Catalog No.:BCC1070
CAS No.:1240299-33-5
- 17 alpha-propionate
Catalog No.:BCC1296
CAS No.:19608-29-8
- Andarine
Catalog No.:BCC1168
CAS No.:401900-40-1
- MDV3100 (Enzalutamide)
Catalog No.:BCC1268
CAS No.:915087-33-1
Quality Control & MSDS
Number of papers citing our products

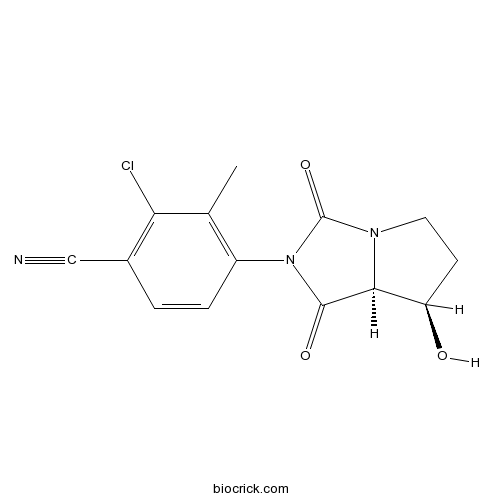
Chemical structure
3D structure
| Cas No. | 627530-84-1 | SDF | Download SDF |
| PubChem ID | 9882972 | Appearance | Powder |
| Formula | C14H12ClN3O3 | M.Wt | 305.72 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 50 mg/mL (163.55 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
| Chemical Name | 4-[(7R,7aS)-7-hydroxy-1,3-dioxo-5,6,7,7a-tetrahydropyrrolo[1,2-c]imidazol-2-yl]-2-chloro-3-methylbenzonitrile | ||
| SMILES | CC1=C(C=CC(=C1Cl)C#N)N2C(=O)C3C(CCN3C2=O)O | ||
| Standard InChIKey | KEJORAMIZFOODM-PWSUYJOCSA-N | ||
| Standard InChI | InChI=1S/C14H12ClN3O3/c1-7-9(3-2-8(6-16)11(7)15)18-13(20)12-10(19)4-5-17(12)14(18)21/h2-3,10,12,19H,4-5H2,1H3/t10-,12+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | BMS-564929 is a highly potent, orally active and nonsteroidal tissue selective modulator of androgen receptor (AR) with Ki value of 2.11 nM. | |||||
| Targets | androgen receptor | |||||
| IC50 | 2.11 nM (Ki) | |||||

BMS-564929 Dilution Calculator

BMS-564929 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.271 mL | 16.3548 mL | 32.7097 mL | 65.4193 mL | 81.7742 mL |
| 5 mM | 0.6542 mL | 3.271 mL | 6.5419 mL | 13.0839 mL | 16.3548 mL |
| 10 mM | 0.3271 mL | 1.6355 mL | 3.271 mL | 6.5419 mL | 8.1774 mL |
| 50 mM | 0.0654 mL | 0.3271 mL | 0.6542 mL | 1.3084 mL | 1.6355 mL |
| 100 mM | 0.0327 mL | 0.1635 mL | 0.3271 mL | 0.6542 mL | 0.8177 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
BMS-564929 is a selective androgen receptor (AR) modulator with Ki value of 2.11 ± 0.16 nM [1].
The AR is a type of nuclear receptor that is activated by the androgenic hormones, testosterone, or dihydrotestosterone. The important function is regulating gene expression.
BMS-564929 is a muscle-tissue specific agonist for AR with a bicyclic hydantoin structure [2]. BMS-564929 is about 400-fold selective for AR vs. PR and more than 1000-fold selective for AR vs. GR, MR and ERα and β. In the C2C12 myoblast cell line, BMS-564929 shows a potency of 0.44 ± 0.03 nM compared with 2.81 ± 0.48 nM measured for testosterone [1].
In castrated male rats, BMS-564929 is substantially more potent than testosterone (T) in promoting the growth of the levator ani muscle, and is highly selective for muscle vs. Prostate. Because of its potent oral activity and tissue selectivity, BMS-564929 is expected to yield beneficial clinical effects in muscle and other tissues with a more favorable safety way [1].
References:
[1]. Ostrowski J, Kuhns JE, Lupisella JA, et al. Pharmacological and x-ray structural characterization of a novel selective androgen receptor modulator: potent hyperanabolic stimulation of skeletal muscle with hypostimulation of prostate in rats. Endocrinology, 2007, 148(1): 4-12.
[2]. Thevis M, Kohler M, Thomas A, et al. Determination of benzimidazole- and bicyclic hydantoin-derived selective androgen receptor antagonists and agonists in human urine using LC-MS/MS. Anal Bioanal Chem, 2008, 391(1): 251-261.
- 4-Amino-N-methylbenzamide
Catalog No.:BCC8685
CAS No.:6274-22-2
- SKF38393 HCl
Catalog No.:BCC6526
CAS No.:62717-42-4
- Dihydrolycorine
Catalog No.:BCN2475
CAS No.:6271-21-2
- NF 449
Catalog No.:BCC7043
CAS No.:627034-85-9
- Dioctanoylglycol
Catalog No.:BCC6662
CAS No.:627-86-1
- H-D-Arg-OH.HCl
Catalog No.:BCC2869
CAS No.:627-75-8
- Saikosaponin F
Catalog No.:BCN2776
CAS No.:62687-63-2
- Handelin
Catalog No.:BCN2953
CAS No.:62687-22-3
- 1-Hydroxy-2-methylanthraquinone
Catalog No.:BCN3478
CAS No.:6268-09-3
- SIB 1893
Catalog No.:BCC6970
CAS No.:6266-99-5
- Ro 106-9920
Catalog No.:BCC7175
CAS No.:62645-28-7
- 11S,12-Dihydroxyspirovetiv-1(10)-en-2-one
Catalog No.:BCN1391
CAS No.:62623-86-3
- SD-208
Catalog No.:BCC1938
CAS No.:627536-09-8
- Epitrametol
Catalog No.:BCN7073
CAS No.:627538-65-2
- Pterophorine
Catalog No.:BCN2118
CAS No.:62786-99-6
- Senampeline A
Catalog No.:BCN2030
CAS No.:62787-00-2
- Senampeline D
Catalog No.:BCN2033
CAS No.:62787-01-3
- SU14813
Catalog No.:BCC1971
CAS No.:627908-92-3
- Palmitic acid ethyl ester
Catalog No.:BCN8298
CAS No.:628-97-7
- Jolkinol A
Catalog No.:BCN3778
CAS No.:62820-11-5
- Meglumine
Catalog No.:BCC4795
CAS No.:6284-40-8
- Senampeline E
Catalog No.:BCN2032
CAS No.:71075-42-8
- 2-Amino-6-nitrobenzothiazole
Catalog No.:BCC8544
CAS No.:6285-57-0
- Senampeline B
Catalog No.:BCN2031
CAS No.:62860-52-0
Determination of benzimidazole- and bicyclic hydantoin-derived selective androgen receptor antagonists and agonists in human urine using LC-MS/MS.[Pubmed:18270691]
Anal Bioanal Chem. 2008 May;391(1):251-61.
Selective androgen receptor modulators (SARMs) represent a novel class of drugs with tissue-specific agonistic and antagonistic properties, which are prohibited in sports from January 2008 according to the World Anti-Doping Agency. Preventive approaches to restrict the use of SARMs include early implementation of target analytes into doping control screening assays. Five model SARMs were synthesized, four of which are analogs to prostate-specific androgen receptor antagonists with a 5,6-dichloro-benzimidazole nucleus. The fifth SARM is a muscle-tissue specific agonist with a bicyclic hydantoin structure (BMS-564929). Dissociation pathways after negative electrospray ionization were studied using an LTQ-Orbitrap mass analyzer, and diagnostic product ions and common fragmentation patterns were employed to establish a screening procedure that target the intact SARMs as well as putative metabolic products. Sample preparation based on solid-phase extraction and subsequent LC-MS/MS measurement allowed for detection limits of 1-20 ng/mL, intra- and interday precisions of between 2.4 and 13.2% and between 6.5 and 24.2%, respectively. Recoveries varied from 89 to 106%, and tests for ion suppression or enhancement effects were negative for all analytes. [figure: see text]
Pharmacological and x-ray structural characterization of a novel selective androgen receptor modulator: potent hyperanabolic stimulation of skeletal muscle with hypostimulation of prostate in rats.[Pubmed:17008401]
Endocrinology. 2007 Jan;148(1):4-12.
A novel, highly potent, orally active, nonsteroidal tissue selective androgen receptor (AR) modulator (BMS-564929) has been identified, and this compound has been advanced to clinical trials for the treatment of age-related functional decline. BMS-564929 is a subnanomolar AR agonist in vitro, is highly selective for the AR vs. other steroid hormone receptors, and exhibits no significant interactions with SHBG or aromatase. Dose response studies in castrated male rats show that BMS-564929 is substantially more potent than testosterone (T) in stimulating the growth of the levator ani muscle, and unlike T, highly selective for muscle vs. prostate. Key differences in the binding interactions of BMS-564929 with the AR relative to the native hormones were revealed through x-ray crystallography, including several unique contacts located in specific helices of the ligand binding domain important for coregulatory protein recruitment. Results from additional pharmacological studies effectively exclude alternative mechanistic contributions to the observed tissue selectivity of this unique, orally active androgen. Because concerns regarding the potential hyperstimulatory effects on prostate and an inconvenient route of administration are major drawbacks that limit the clinical use of T, the potent oral activity and tissue selectivity exhibited by BMS-564929 are expected to yield a clinical profile that provides the demonstrated beneficial effects of T in muscle and other tissues with a more favorable safety window.
Detection of SARMs in doping control analysis.[Pubmed:28137616]
Mol Cell Endocrinol. 2018 Mar 15;464:34-45.
The class of selective androgen receptor modulators (SARMs) has been the subject of intense and dedicated clinical research over the past two decades. Potential therapeutic applications of SARMs are manifold and focus particularly on the treatment of conditions manifesting in muscle loss such as general sarcopenia, cancer-associated cachexia, muscular dystrophy, etc. Consequently, based on the substantial muscle- and bone-anabolic properties of SARMs, these agents constitute substances with significant potential for misuse in sport and have therefore been added to the Word Anti-Doping Agency's (WADA's) Prohibited List in 2008. Since then, numerous adverse analytical findings have been reported for various different SARMs, which has underlined the importance of proactive and preventive anti-doping measures concerning emerging drugs such as these anabolic agents, which have evidently been misused in sport despite the fact that none of these SARMs has yet received full clinical approval. In this review, analytical data on SARMs generated in the context of research conducted for sports drug testing purposes are summarized and state-of-the-art test methods aiming at intact drugs as well as diagnostic urinary metabolites are discussed. Doping control analytical approaches predominantly rely on chromatography hyphenated to mass spectrometry, which have allowed for appropriately covering the considerable variety of pharmacophores present in SARMs such as the non-steroidal representatives ACP-105, BMS-564929, GLPG0492 (DT-200), LG-121071, LGD-2226, LGD-4033/VK 5211, ostarine/enobosarm, RAD-140, S-40503, etc. as well as steroidal compounds such as MK-0773 and YK-11.


