BHQinhibitor of endoplasmic reticulum Ca2+-ATPase CAS# 88-58-4 |
- Regorafenib monohydrate
Catalog No.:BCC1884
CAS No.:1019206-88-2
- AZD4547
Catalog No.:BCC3711
CAS No.:1035270-39-3
- LY2874455
Catalog No.:BCC1723
CAS No.:1254473-64-7
- NVP-BGJ398 phosphate
Catalog No.:BCC1814
CAS No.:1310746-10-1
- Nintedanib (BIBF 1120)
Catalog No.:BCC3661
CAS No.:656247-17-5
- BIBF 1202
Catalog No.:BCC5298
CAS No.:894783-71-2
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 88-58-4 | SDF | Download SDF |
| PubChem ID | 2374 | Appearance | Powder |
| Formula | C14H22O2 | M.Wt | 222.33 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO | ||
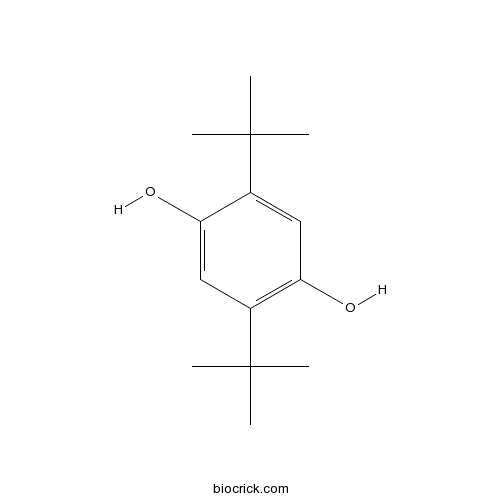
| Chemical Name | 2,5-ditert-butylbenzene-1,4-diol | ||
| SMILES | CC(C)(C)C1=CC(=C(C=C1O)C(C)(C)C)O | ||
| Standard InChIKey | JZODKRWQWUWGCD-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C14H22O2/c1-13(2,3)9-7-12(16)10(8-11(9)15)14(4,5)6/h7-8,15-16H,1-6H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | A selective inhibitor of endoplasmic reticulum Ca2+-ATPase. |

BHQ Dilution Calculator

BHQ Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.4978 mL | 22.4891 mL | 44.9782 mL | 89.9564 mL | 112.4455 mL |
| 5 mM | 0.8996 mL | 4.4978 mL | 8.9956 mL | 17.9913 mL | 22.4891 mL |
| 10 mM | 0.4498 mL | 2.2489 mL | 4.4978 mL | 8.9956 mL | 11.2445 mL |
| 50 mM | 0.09 mL | 0.4498 mL | 0.8996 mL | 1.7991 mL | 2.2489 mL |
| 100 mM | 0.045 mL | 0.2249 mL | 0.4498 mL | 0.8996 mL | 1.1245 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
BHQ is a selective inhibitor of endoplasmic reticulum Ca2+-ATPase.
Endoplasmic reticulum Ca2+-ATPase (SR Ca2+-ATPase) is a Ca2+-ATPase and transfers Ca2+ from the cytosol of the cell to the lumen of the sarcoplasmic reticulum (SR) during muscle relaxation.
BHQ is a selective SR Ca2+-ATPase inhibitor. In rat basophilic leukaemia cells, BHQ (10 μM) blocked inward rectifier potassium current and might cause depolarization of the cell and affect Ca2+ influx [1]. In aortic rings at rest or depolarised with 80 mM K+ in the presence of 1 mM nifedipine, BHQ induced a slow tonic contraction. At 20 mM K+, BHQ increased Ca2+-induced contraction. However, BHQ inhibited Ca2+-induced contraction at 40, 80 and 128 mM K+ [2]. In smooth muscle cells from the rat tail artery, BHQ reduced L-type Ca2+ current (ICa(L)) with IC50 value of 66.7 μM in a concentration- and voltage-dependent way. BHQ increased superoxide anion formation, which was markedly inhibited by superoxide dismutase (SOD). These results suggested that BHQ inhibited ICa(L) by the generation of superoxide anion [3]. In Madin Darby canine kidney (MDCK) cells, BHQ increased [Ca2+]i with EC50 value of 40 μM in a dose-dependent way, which was contributed by depleting the endoplasmic reticulum Ca2+ store followed by capacitative Ca2+ entry [4].
References:
[1]. Hasséssian H, Vaca L, Kunze DL. Blockade of the inward rectifier potassium current by the Ca(2+)-ATPase inhibitor 2',5'-di(tert-butyl)-1,4-benzohydroquinone (BHQ). Br J Pharmacol, 1994, 112(4): 1118-1122.
[2]. Fusi F, Gorelli B, Valoti M, et al. Effects of 2,5-di-t-butyl-1,4-benzohydroquinone (BHQ) on rat aorta smooth muscle. Eur J Pharmacol, 1998, 346(2-3): 237-243.
[3]. Fusi F, Saponara S, Gagov H, et al. 2,5-Di-t-butyl-1,4-benzohydroquinone (BHQ) inhibits vascular L-type Ca(2+) channel via superoxide anion generation. Br J Pharmacol, 2001, 133(7): 988-996.
[4]. Jan CR, Ho CM, Wu SN, et al. Mechanism of rise and decay of 2,5-di-tert-butylhydroquinone-induced Ca2+ signals in Madin Darby canine kidney cells. Eur J Pharmacol, 1999, 365(1): 111-117.
- 2-Acetylthiophene
Catalog No.:BCC8517
CAS No.:88-15-3
- Furan-2-carboxylic acid
Catalog No.:BCN4557
CAS No.:88-14-2
- Ingenol-5,20-acetonide-3-O-angelate
Catalog No.:BCN8458
CAS No.:87980-68-5
- UFP 803
Catalog No.:BCC5917
CAS No.:879497-82-2
- Prionoid E
Catalog No.:BCN3161
CAS No.:879324-78-4
- Prionoid D
Catalog No.:BCN3160
CAS No.:879324-77-3
- Prionoid C
Catalog No.:BCN3159
CAS No.:879324-76-2
- Prionoid B
Catalog No.:BCN3217
CAS No.:879324-75-1
- p-Hydroxyphenethyl anisate
Catalog No.:BCN6896
CAS No.:87932-34-1
- 3,4,5-Tri-O-galloylquinic acid
Catalog No.:BCN8027
CAS No.:99745-62-7
- Psoracorylifol C
Catalog No.:BCN3708
CAS No.:879290-99-0
- Psoracorylifol B
Catalog No.:BCN7884
CAS No.:879290-98-9
- 1,2-Benzenedicarboxylic acid
Catalog No.:BCN3151
CAS No.:88-99-3
- Neuropathiazol
Catalog No.:BCC5375
CAS No.:880090-88-0
- Fmoc-Hyp-OH
Catalog No.:BCC3254
CAS No.:88050-17-3
- AMD-070 hydrochloride
Catalog No.:BCC1358
CAS No.:880549-30-4
- Naloxonazine dihydrochloride
Catalog No.:BCC6710
CAS No.:880759-65-9
- PhiKan 083
Catalog No.:BCC2411
CAS No.:880813-36-5
- 4-Hydroxybenzaldehyde rhamnoside
Catalog No.:BCN7625
CAS No.:88086-86-6
- Phrixotoxin 3
Catalog No.:BCC6328
CAS No.:880886-00-0
- Notoginsenoside Fa
Catalog No.:BCN3854
CAS No.:88100-04-3
- HDS 029
Catalog No.:BCC7441
CAS No.:881001-19-0
- 30-Hydroxygambogic acid
Catalog No.:BCN3081
CAS No.:881027-36-7
- Notoginsenoside Fe
Catalog No.:BCN3852
CAS No.:88105-29-7
t-BHQ Provides Protection against Lead Neurotoxicity via Nrf2/HO-1 Pathway.[Pubmed:26798413]
Oxid Med Cell Longev. 2016;2016:2075915.
The neurotoxicity of lead has been well established, and oxidative stress is strongly associated with lead-induced neurotoxicity. Nrf2 is important for protection against oxidative stress in many disease models. We applied t-BHQ, which is an Nrf2 activator, to investigate the possible role of Nrf2 in the protection against lead neurotoxicity. t-BHQ significantly attenuated the oxidative stress in developmental rats by decreasing MDA level, as well as by increasing SOD activity and GSH content, in the hippocampus and frontal cortex. Furthermore, neuronal apoptosis was detected by Nissl staining, and Bax expression was inhibited in the t-BHQ-treated group. Results showed that t-BHQ suppressed ROS production and caspase 3/7 activity but increased intracellular GSH content, in SH-SY5Y cells under lead exposure. Moreover, in vivo and in vitro, t-BHQ enhanced the nuclear translocation of Nrf2 and binding to ARE areas but did not induce Nrf2 transcription. These phenomena were confirmed using RT-PCR, EMSA, Western blot, and immunofluorescence analyses. Subsequent upregulation of the expression of HO-1, NQO1, and GCLC was observed. However, knockdown of Nrf2 or HO-1 adversely affected the protective effects of t-BHQ against lead toxicity in SH-SY5Y cells. Thus, t-BHQ can protect against lead neurotoxicity, depending on the Nrf2/HO-1 pathway.
Synthesis, in vitro evaluation, and in vivo metabolism of fluor/quencher compounds containing IRDye 800CW and Black Hole Quencher-3 (BHQ-3).[Pubmed:21639144]
Bioconjug Chem. 2011 Jul 20;22(7):1287-97.
Protease-cleavable peptides containing a suitable fluor/quencher (Fl/Q) pair are optically dark until cleaved by their target protease, generating fluorescence. This approach has been used with many Fl/Q pairs, but little has been reported with IRDye 800CW, a popular near-infrared (NIR) fluor. We explored the use of the azo-bond-containing Black Hole Quencher 3 (BHQ-3) as a quencher for IRDye 800CW and found that IRDye 800CW/BHQ-3 is a suitable Fl/Q pair, despite the lack of proper spectral overlap for fluorescence resonance energy transfer (FRET) applications. Cleavage of IRDye 800CW-PLGLK(BHQ-3)AR-NH(2) (8) and its D-arginine (Darg) analogue (9) by matrix metalloproteinases (MMPs) in vitro yielded the expected cleavage fragments. In vivo, extensive metabolism was found. Significant decomposition of a "non-cleavable" control IRDye 800CW-(1,13-diamino-4,7,10-trioxatridecane)-BHQ-3 (10) was evident in plasma of normal mice by 3 min post injection. The major metabolite showed a m/z and UV/vis spectrum consistent with azo bond cleavage in the BHQ-3 moiety. Preparation of an authentic standard of this metabolite (11) confirmed the assignment. Although the IRDye 800CW/BHQ-3 constructs showed efficient contact quenching prior to enzymatic cleavage, BHQ-3 should be used with caution in vivo, due to instability of its azo bond.
[Effect of TMB-8 on the increase of intracellular free Ca2+ induced by NE and BHQ in dissociated single rat brain cell].[Pubmed:11596212]
Yao Xue Xue Bao. 1997 Oct;32(10):726-30.
The inhibitory effect and mechanism of 8-(N, N'-diethylamino) octyl 3, 4, 5-trimethoxybenzoate hydrochloride (TMB-8) on the elevation of single intracellular free Ca2+ concentration ([Ca2+]i) induced by High K+, Norepinephrine(NE) and 2, 5-Di(tert-butyl)-1, 4-benzohydroquinone (BHQ) in dissociated single rat brain cells were studied. The changes of [Ca2+]i were reflected by the fluorescent indicator, Fura-2/AM, employed. In the absence of extracellular Ca2+, Ca-free Hank's solution, preincubation with TMB-8 (10, 30 mumol.L-1) for 20 min significantly decreased the resting [Ca2+]i from 79 +/- 13 nmol.L-1 to 65 +/- 11 and 61 +/- 6 nmol.L-1, respectively. [Ca2+]i were markedly increased by NE and BHQ and reduced significantly to control level by TMB-8. On the other hand, when the cells were incubated in Hank's solution containing Ca2+ 1.3 mmol.L-1, TMB-8(30, 100 mumol.L-1) suppressed the increase of [Ca2+]i induced by NE (0.0001-0.1 mumol.L-1). TMB-8 showed no significant effect on [Ca2+]i elevation induced by KCl and BHQ in Hank's solution containing Ca2+ 1.3 mmol.L-1. These results indicate that TMB-8 reduced [Ca2+]i via increase of the sarcoplasmic reticulum (SR) sequestration, which blocked the release of intracellular store from the SR. However, the inhibitory effect of TMB-8 on Ca-influx from extracellular medium seems to be an indirect action from the saturation of SR with calcium.
[8-(N,N-diethylamino)-n-octyl-3,4,5-trimethoxybenzoate(TMB-8) reduced the elevation of [Ca2+]i induced by BHQ, NE and KCl in cultured single smooth muscle cells of the calf basilar artery].[Pubmed:11596200]
Yao Xue Xue Bao. 1997 Nov;32(11):819-23.
The effect of 8-(N, N-diethylamino)-n-octyl-3,4,5-trimethoxybenzoate (TMB-8) on the elevation of [Ca2+]i induced by 2, 5-di (tert-butyl)-1, 4-benzohydroquinone (BHQ), norepinephrine (NE), KCl in cultured single smooth muscle cells of the calf basilar artery was studied by a system of measurement of AR-CM-MIC, using Fura-2/AM as a fluoresent indicator. In the presence of extracellular Ca2+ 1.3 mmol.L-1, the resting [Ca2+]i was not changed by TMB-8 (10, 30 and 100 mumol.L-1), but the elevation of [Ca2+]i induced by BHQ, NE and KCl were reduced by TMB-8 (30 mumol.L-1) significantly. In Ca2+ free Hank's solution containing EGTA 0.1 mmol.L-1, the resting [Ca2+]i was markedly reduced by TMB-8 (10, 30 and 100 mumol.L-1), and the increase of [Ca2+]i evoked by BHQ and NE was blocked completely by TMB-8 (30 mumol.L-1). The result suggested that TMB-8 inhibited the Ca2+ release from intracellular stores or increased the up-take of Ca2+ into sarcoplasmic reticulum and the inhibition of Ca(2+)-influx from extracellular site may be an indirect machanism.
Effects of three sarcoplasmic/endoplasmic reticulum Ca++ pump inhibitors on release channels of intracellular stores.[Pubmed:9580621]
J Pharmacol Exp Ther. 1998 May;285(2):739-45.
The three principal sarcoplasmic/endoplasmic reticulum Ca++ pump inhibitors have been compared for their effects on Ca++ fluxes across intracellular stores present in isolated skeletal muscle and brain membrane preparations. At moderate concentrations that only partially inhibited Ca++ pumping, all three inhibitors induced transient release of Ca++ from isolated sarcoplasmic reticulum membranes, and release was ruthenium red-sensitive, much faster and sustained at higher pump inhibitor concentrations. In contrast, in unidirectional 45Ca efflux assays, cyclopiazonic acid appeared to have little effect, thapsigargin decreased efflux and 2,5-di(tert-butyl)-1,4-benzohydroquinone increased efflux only slightly. These observations taken together suggest that transient releases were manifest primarily by vesicles with a high ratio of ryanodine receptors to pumps (and thus more susceptible to becoming leaky with only some pumps inhibited), and that Ca(++)-induced Ca++ release amplified releases when all pumps were blocked. These mostly indirect side effects were specific for ryanodine receptors. In similar experiments with brain cerebellar membranes, none of the three inhibitors appeared to directly reduce release induced by inositol 1,4,5-trisphosphate. These findings may affect interpretation of results of experiments involving application of these compounds to isolated membranes, cells or tissue preparations.
2,5-Di-t-butyl-1,4-benzohydroquinone induces endothelium-dependent relaxation of rat thoracic aorta.[Pubmed:10082199]
Eur J Pharmacol. 1999 Feb 5;366(2-3):181-7.
The aim of this work was to clarify the mechanism by which 2,5-di-t-butyl-1,4-benzohydroquinone (BHQ) induces relaxation of rat thoracic aorta. In particular, the role of endothelium-derived nitric oxide (NO) was investigated. BHQ concentration dependently (0.1-10 microM) relaxed rat aorta rings precontracted with phenylephrine. This effect was dependent on the intactness of the endothelium, suppressed by preincubation with 100 microM N(omega)-nitro-L-arginine methyl ester and antagonised by 3-30 microM methylene blue. The 10 microM BHQ-induced relaxation, however, was followed by the gradual and slow return to phenylephrine-induced tone. Superoxide dismutase (250 U/ml) increased the BHQ-induced relaxation, while preincubation with 3 mM diethyldithiocarbamate inhibited it in a time-dependent fashion. BHQ gave rise to superoxide anion formation which was markedly inhibited by the addition of superoxide dismutase (250 U/ml), either in the presence or in the absence of aorta rings. The non-specific blocker of Ca2+ channels, Ni2+, concentration dependently attenuated the BHQ relaxing effect. BHQ did not modify the relaxation induced by the NO donor 3-morpholino-sydnonimine in endothelium-deprived rings. In conclusion, BHQ induces endothelium-dependent relaxation and gives rise, by auto-oxidation, to the formation of superoxide anion. The former effect results from the enhanced synthesis of NO rather than from its enhanced biological activity; NO synthase is presumed to be stimulated by BHQ-induced activation of Ca2+ influx through Ni2+-sensitive Ca2+ channels.
Mechanism of rise and decay of 2,5-di-tert-butylhydroquinone-induced Ca2+ signals in Madin Darby canine kidney cells.[Pubmed:9988129]
Eur J Pharmacol. 1999 Jan 15;365(1):111-7.
We examined the effect of 2,5-di-tert-butylhydroquinone (BHQ) on intracellular Ca2+ concentrations ([Ca2+]i) measured by fura-2 fluorimetry in Madin Darby canine kidney (MDCK) cells. BHQ increased [Ca2+]i in a dose-dependent manner with an EC50 of 40 microM. The Ca2+ signal showed a slow onset, a gradual decay and a sustained plateau in normal Ca2+ medium. Depletion of the endoplasmic reticulum Ca2+ store by incubation with 0.1 mM BHQ for 6 min abolished the [Ca2+]i increase evoked by bradykinin or ATP, suggesting that BHQ depleted the inositol 1,4,5-trisphosphate (IP3)-sensitive Ca2+ store. Removal of extracellular Ca2+ reduced the BHQ response by 50%. The Ca2+ signal was initiated by Ca2+ release from the internal store, followed by capacitative Ca2+ entry which was abolished by 100 microM La3+ or 50 microM Gd3+ and was partly inhibited by 1-[beta-[3-(4-methoxyphenyl)propoxy]-4-methoxyphenethyl]-1H-imidazole hydrochloride (SKF 96365). After depletion of the endoplasmic reticulum Ca2+ store, by incubation with another inhibitor of the endoplasmic reticulum Ca2+ pump, thapsigargin for 30 min, BHQ did not elevate [Ca2+]i, suggesting that the BHQ-induced Ca2+ influx was largely due to capacitative Ca2+ entry, and that BHQ released Ca2+ from the thapsigargin-sensitive endoplasmic reticulum store. We investigated the mechanism of decay of the BHQ response. Pretreatemt with La3+ (or Gd3+) or alkalization of the extracellular medium to pH 8 significantly potentiated the Ca2+ signal, whereas pretreatment with carbonylcyanide m-chlorophenylhydrazone (CCCP) or oligomycin, or removal of extracellular Na+, had no effect. Collectively, our results suggest that BHQ increased [Ca2+]i in MDCK cells by depleting the endoplasmic reticulum Ca2+ store followed by capacitative Ca2+ entry, with both pathways contributing equally. The decay of the BHQ response is effected by Ca2+ efflux via the plasma membrane Ca2+ pump, but not by efflux via Na+/Ca2+ exchange or sequestration by the mitochondria.
Blockade of the inward rectifier potassium current by the Ca(2+)-ATPase inhibitor 2',5'-di(tert-butyl)-1,4-benzohydroquinone (BHQ).[Pubmed:7952872]
Br J Pharmacol. 1994 Aug;112(4):1118-22.
1. We have investigated the effect of 2',5'-di (tert-butyl)-1,4-benzohydroquinone (BHQ) and thapsigargin, inhibitors of the intracellular Ca(2+)-ATPase, on ionic currents in rat basophilic leukaemia (RBL-2H3) cells under whole cell voltage clamp. 2. The whole cell current was inwardly rectifying and reversed at -35 +/- 6 mV (n = 16). The conductance of the inward current increased as the concentration of extracellular K+ was raised from 2.7 to 5.4, 10.8 and 21.6 mM. BaCl2 (100 microM) reduced the current to a small linear component and shifted the reversal potential to -4 +/- 3 mV (n = 6). A concentration of 50 microM BaCl2 produced 45 +/- 10% (n = 4) blockade of the inward current. 3. BHQ and thapsigargin were examined for their effects on the inwardly rectifying current. A maximal blockade of inward current was obtained within 6 min after perfusion with 10 microM BHQ. The small current remaining after blockade with BHQ had a linear voltage-dependence and reversed direction at -6 +/- 9 mV (n = 6). Thapsigargin (up to 3 microM) was without effect on the inward rectifier. 4. In contrast to the blockade of the inward rectifier produced by BaCl2 which was predominantly on the steady state current, particularly at the very hyperpolarized holding potentials (-120 mV), blockade by BHQ was equally strong on the instantaneous as well as the steady state current. 5. Blockade of the inward rectifier by BHQ may cause depolarization of the cell which will affect Ca2+ influx during investigations with BHQ. Thapsigargin does not block the inward rectifier and will not inhibit Ca2+ influx in this way.


