BETPPositive allosteric modulator of GLP-1 receptors CAS# 1371569-69-5 |
- Amyloid β-Peptide (10-20) (human)
Catalog No.:BCC1026
CAS No.:152286-31-2
- Amyloid β-Protein (1-15)
Catalog No.:BCC1003
CAS No.:183745-81-5
- Beta-Amyloid (1-11)
Catalog No.:BCC1002
CAS No.:190436-05-6
- Myelin Basic Protein (68-82), guinea pig
Catalog No.:BCC1020
CAS No.:98474-59-0
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 1371569-69-5 | SDF | Download SDF |
| PubChem ID | 49868481 | Appearance | Powder |
| Formula | C20H17F3N2O2S | M.Wt | 406.42 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 100 mg/mL (246.05 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
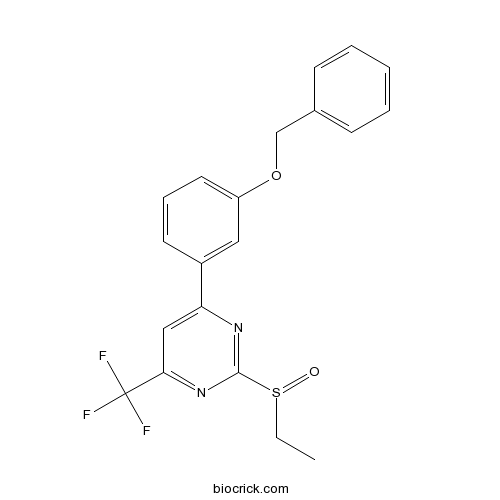
| Chemical Name | 2-ethylsulfinyl-4-(3-phenylmethoxyphenyl)-6-(trifluoromethyl)pyrimidine | ||
| SMILES | CCS(=O)C1=NC(=CC(=N1)C(F)(F)F)C2=CC(=CC=C2)OCC3=CC=CC=C3 | ||
| Standard InChIKey | NTDFYGSSDDMNHI-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C20H17F3N2O2S/c1-2-28(26)19-24-17(12-18(25-19)20(21,22)23)15-9-6-10-16(11-15)27-13-14-7-4-3-5-8-14/h3-12H,2,13H2,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective positive allosteric modulator and partial agonist of the glucagon-like peptide 1 (GLP-1) receptor. Increases binding affinity of oxyntomodulin for the GLP-1 receptor. Potentiates oxyntomodulin-mediated GLP-1 receptor signaling in vitro and insulin secretion in vivo. Has no effect on GLP-2, GIP, PTH or glucagon receptors. |

BETP Dilution Calculator

BETP Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4605 mL | 12.3025 mL | 24.6051 mL | 49.2102 mL | 61.5127 mL |
| 5 mM | 0.4921 mL | 2.4605 mL | 4.921 mL | 9.842 mL | 12.3025 mL |
| 10 mM | 0.2461 mL | 1.2303 mL | 2.4605 mL | 4.921 mL | 6.1513 mL |
| 50 mM | 0.0492 mL | 0.2461 mL | 0.4921 mL | 0.9842 mL | 1.2303 mL |
| 100 mM | 0.0246 mL | 0.123 mL | 0.2461 mL | 0.4921 mL | 0.6151 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- (±)-Marmesin
Catalog No.:BCN3618
CAS No.:13710-70-8
- Tolfenamic Acid
Catalog No.:BCC4438
CAS No.:13710-19-5
- Pimecrolimus
Catalog No.:BCC4703
CAS No.:137071-32-0
- Alprenolol hydrochloride
Catalog No.:BCC7490
CAS No.:13707-88-5
- PACAP 1-38
Catalog No.:BCC6962
CAS No.:137061-48-4
- Walsuralactam A
Catalog No.:BCN6734
CAS No.:1370556-82-3
- Episyringaresinol 4'-O-β-D-glncopyranoside
Catalog No.:BCC8957
CAS No.:137038-13-2
- Pseudoginsenoside Rh2
Catalog No.:BCC8353
CAS No.:1370264-16-6
- PRT062607 Hydrochloride
Catalog No.:BCC1869
CAS No.:1370261-97-4
- 3',4'-Di-O-acetyl-2',6'-di-O-p-coumaroylastragalin
Catalog No.:BCN6610
CAS No.:137018-33-8
- Amprolium HCl
Catalog No.:BCC4626
CAS No.:137-88-2
- L-Ascorbyl 6-palmitate
Catalog No.:BCC4915
CAS No.:137-66-6
- Toddalosin
Catalog No.:BCN6194
CAS No.:137182-37-7
- Spinorphin
Catalog No.:BCC2349
CAS No.:137201-62-8
- Sodium Orthovanadate
Catalog No.:BCC3856
CAS No.:13721-39-6
- Voriconazole
Catalog No.:BCC2275
CAS No.:137234-62-9
- Dodoviscin A
Catalog No.:BCN3927
CAS No.:1372527-25-7
- Dodoviscin H
Catalog No.:BCN3918
CAS No.:1372527-39-3
- Dodoviscin I
Catalog No.:BCN3926
CAS No.:1372527-40-6
- Dodoviscin J
Catalog No.:BCN3945
CAS No.:1372527-42-8
- GSK2636771
Catalog No.:BCC4993
CAS No.:1372540-25-4
- Chlorantholide A
Catalog No.:BCN4835
CAS No.:1372558-33-2
- Chlorantholide B
Catalog No.:BCN4834
CAS No.:1372558-34-3
- Chlorantholide C
Catalog No.:BCN4837
CAS No.:1372558-35-4
Substrate-bound outward-open state of the betaine transporter BetP provides insights into Na+ coupling.[Pubmed:25023443]
Nat Commun. 2014 Jul 15;5:4231.
The Na(+)-coupled betaine symporter BETP shares a highly conserved fold with other sequence unrelated secondary transporters, for example, with neurotransmitter symporters. Recently, we obtained atomic structures of BETP in distinct conformational states, which elucidated parts of its alternating-access mechanism. Here, we report a structure of BETP in a new outward-open state in complex with an anomalous scattering substrate, adding a fundamental piece to an unprecedented set of structural snapshots for a secondary transporter. In combination with molecular dynamics simulations these structural data highlight important features of the sequential formation of the substrate and sodium-binding sites, in which coordinating water molecules play a crucial role. We observe a strictly interdependent binding of betaine and sodium ions during the coupling process. All three sites undergo progressive reshaping and dehydration during the alternating-access cycle, with the most optimal coordination of all substrates found in the closed state.
Regulatory role of charged clusters in the N-terminal domain of BetP from Corynebacterium glutamicum.[Pubmed:26146128]
Biol Chem. 2015 Sep;396(9-10):1117-26.
The trimeric transporter BETP counteracts hyperosmotic stress by a fast increase in transport rate in order to accumulate the compatible solute betaine. The positively charged alpha-helical C-terminal domain acts as an osmosensor perceiving the increase in the internal potassium (K+) concentration. A second, still unidentified stimulus originates from stress-induced changes in the physical state of the membrane and depends on the amount of negatively charged lipids. BETP possesses a 60-amino acid (aa)-long negatively charged N-terminal domain, which is predicted to adopt a partly helical fold affecting osmoregulation by an unknown mechanism. It is assumed that the C-terminal domain, the N-terminal domain, and negatively charged lipids interact during stress sensing and regulation. Here, we have investigated the regulatory role of negatively charged clusters in the N-terminal domain. We identified one cluster, Glu24Glu25, to be crucial for osmoregulation. Cross-linking studies revealed an interaction between the C- and N-terminal domains of adjacent protomers modulating transport activation. A regulatory partner-switching mechanism emerges in which the C-terminal domain changes its interaction with the N-terminal domain of its own promoter and negatively charged lipids to an interaction with the N-terminal domain of an adjacent protomer and lipids bound to the central cavity of the BETP trimer.
Analysis of putative protomer crosstalk in the trimeric transporter BetP: The heterotrimer approach.[Pubmed:24637177]
Biochim Biophys Acta. 2014 Jun;1837(6):888-98.
The homotrimeric, secondary active betaine carrier BETP from Corynebacterium glutamicum is a model system for stress-regulated transport in bacteria. Its activity responds to hyperosmotic stress and it harbors two different functions, transport catalysis (betaine uptake) and stimulus sensing, resp. activity regulation. Structural information from 2D and 3D crystals as well as functional analysis of monomerized BETP suggested the presence of conformational crosstalk between the individual protomers. To study whether the oligomeric state is functionally significant on a mechanistic level we generated heterooligomeric complexes of BETP in which single protomers within the trimer can be addressed. By testing dominant negative effects in a trimer of one active protomer combined with two protomers in which transport and regulation were abolished, we provide experimental evidence for the absence of functionally significant conformational crosstalk between the protomers on the level of both transport and regulation. This is supported by experiments using mutant forms of putative interacting signal donor and acceptor domains of individual BETP protomers. This result has important consequences for oligomeric transport proteins in general and BETP in particular.
Lipid-Protein Interactions in the Regulated Betaine Symporter BetP Probed by Infrared Spectroscopy.[Pubmed:26592930]
J Biol Chem. 2016 Feb 26;291(9):4295-307.
The Na(+)-coupled betaine symporter BETP senses changes in the membrane state and increasing levels of cytoplasmic K(+) during hyperosmotic stress latter via its C-terminal domain and regulates transport activity according to both stimuli. This intriguing sensing and regulation behavior of BETP was intensively studied in the past. It was shown by several biochemical studies that activation and regulation depends crucially on the lipid composition of the surrounding membrane. In fact, BETP is active and regulated only when negatively charged lipids are present. Recent structural studies have revealed binding of phosphatidylglycerol lipids to functional important parts of BETP, suggesting a functional role of lipid interactions. However, a regulatory role of lipid interactions could only be speculated from the snapshot provided by the crystal structure. Here, we investigate the nature of lipid-protein interactions of BETP reconstituted in closely packed two-dimensional crystals of negatively charged lipids and probed at the molecular level with Fourier transform infrared (FTIR) spectroscopy. The FTIR data indicate that K(+) binding weakens the interaction of BETP especially with the anionic lipid head groups. We suggest a regulation mechanism in which lipid-protein interactions, especially with the C-terminal domain and the functional important gating helices transmembrane helice 3 (TMH3) and TMH12, confine BETP to its down-regulated transport state. As BETP is also activated by changes in the physical state of the membrane, our results point toward a more general mechanism of how active transport can be modified by dynamic lipid-protein interactions.
Two small molecule agonists of glucagon-like peptide-1 receptor modulate the receptor activation response differently.[Pubmed:22177947]
Biochem Biophys Res Commun. 2012 Jan 6;417(1):558-63.
The glucagon-like peptide-1 receptor (GLP-1R) is a target for type 2 diabetes treatment. Due to the inconvenience of peptide therapeutics, small-molecule GLP-1R agonists have been studied. Compound 2 (6,7-dichloro-2-methylsulfonyl-2-N-tert-butylaminoquinoxaline) and compound B (4-(3-(benzyloxy)phenyl)-2-(ethylsulfinyl)-6-(trifluoromethyl)pyrimidine) have been described as small molecule, ago-allosteric modulators of GLP-1R. However, their modes of action at the GLP-1R have not been elucidated. Thus, in this study, we compared the mechanisms of action between these two compounds. When compound 2 was treated with endogenous or exogenous peptide agonists (GLP-1 and exenatide) or fragments of peptide agonists (GLP-1(9-36), Ex3, Ex4, and Ex5), the response curve of these peptide agonists shifted left without a change in maximum efficacy. In contrast, compound B potentiated the response and increased maximum efficacy. However, N-terminal truncated orthosteric antagonists including Ex7, Ex9, and Ex10, augmented the response of compound 2 at the GLP-1R but did not alter compound B activity. Intriguingly, when we co-treated compound 2 with compound B in CHO cells expressing full-length hGLP-1R or N-terminal extracellular domain-truncated GLP-1R, the activation of both types of receptors increased additively, implying that the N-terminus of the receptor is not involved in the modulation by compound agonists. We confirmed that these two compounds increased calcium influx by different patterns in CHO cells expressing GLP-1R. Taken together, our findings suggest that compounds 2 and B have different modes of action to activate GLP-1R. Further study to identify the putative binding sites will help in the discovery of orally available GLP-1R agonists.
Small molecule allosteric modulation of the glucagon-like Peptide-1 receptor enhances the insulinotropic effect of oxyntomodulin.[Pubmed:22930710]
Mol Pharmacol. 2012 Dec;82(6):1066-73.
Identifying novel mechanisms to enhance glucagon-like peptide-1 (GLP-1) receptor signaling may enable nascent medicinal chemistry strategies with the aim of developing new orally available therapeutic agents for the treatment of type 2 diabetes mellitus. Therefore, we tested the hypothesis that selectively modulating the low-affinity GLP-1 receptor agonist, oxyntomodulin, would improve the insulin secretory properties of this naturally occurring hormone to provide a rationale for pursuing an unexplored therapeutic approach. Signal transduction and competition binding studies were used to investigate oxyntomodulin activity on the GLP-1 receptor in the presence of the small molecule GLP-1 receptor modulator, 4-(3-benzyloxyphenyl)-2-ethylsulfinyl-6-(trifluoromethyl)pyrimidine (BETP). In vivo, the intravenous glucose tolerance test characterized oxyntomodulin-induced insulin secretion in animals administered the small molecule. BETP increased oxyntomodulin binding affinity for the GLP-1 receptor and enhanced oxyntomodulin-mediated GLP-1 receptor signaling as measured by activation of the alpha subunit of heterotrimeric G protein and cAMP accumulation. In addition, oxyntomodulin-induced insulin secretion was enhanced in the presence of the compound. BETP was pharmacologically characterized to induce biased signaling by oxyntomodulin. These studies demonstrate that small molecules targeting the GLP-1 receptor can increase binding and receptor activation of the endogenous peptide oxyntomodulin. The biased signaling engendered by BETP suggests that GLP-1 receptor mobilization of cAMP is the critical insulinotropic signaling event. Because of the unique metabolic properties of oxyntomodulin, identifying molecules that enhance its activity should be pursued to assess the efficacy and safety of this novel mechanism.
Novel small molecule glucagon-like peptide-1 receptor agonist stimulates insulin secretion in rodents and from human islets.[Pubmed:20823098]
Diabetes. 2010 Dec;59(12):3099-107.
OBJECTIVE: The clinical effectiveness of parenterally-administered glucagon-like peptide-1 (GLP-1) mimetics to improve glucose control in patients suffering from type 2 diabetes strongly supports discovery pursuits aimed at identifying and developing orally active, small molecule GLP-1 receptor agonists. The purpose of these studies was to identify and characterize novel nonpeptide agonists of the GLP-1 receptor. RESEARCH DESIGN AND METHODS: Screening using cells expressing the GLP-1 receptor and insulin secretion assays with rodent and human islets were used to identify novel molecules. The intravenous glucose tolerance test (IVGTT) and hyperglycemic clamp characterized the insulinotropic effects of compounds in vivo. RESULTS: Novel low molecular weight pyrimidine-based compounds that activate the GLP-1 receptor and stimulate glucose-dependent insulin secretion are described. These molecules induce GLP-1 receptor-mediated cAMP signaling in HEK293 cells expressing the GLP-1 receptor and increase insulin secretion from rodent islets in a dose-dependent manner. The compounds activate GLP-1 receptor signaling, both alone or in an additive fashion when combined with the endogenous GLP-1 peptide; however, these agonists do not compete with radiolabeled GLP-1 in receptor-binding assays. In vivo studies using the IVGTT and the hyperglycemic clamp in Sprague Dawley rats demonstrate increased insulin secretion in compound-treated animals. Further, perifusion assays with human islets isolated from a donor with type 2 diabetes show near-normalization of insulin secretion upon compound treatment. CONCLUSIONS: These studies characterize the insulinotropic effects of an early-stage, small molecule GLP-1 receptor agonist and provide compelling evidence to support pharmaceutical optimization.


