AlbonoursinCAS# 1222-90-8 |
Quality Control & MSDS
Number of papers citing our products

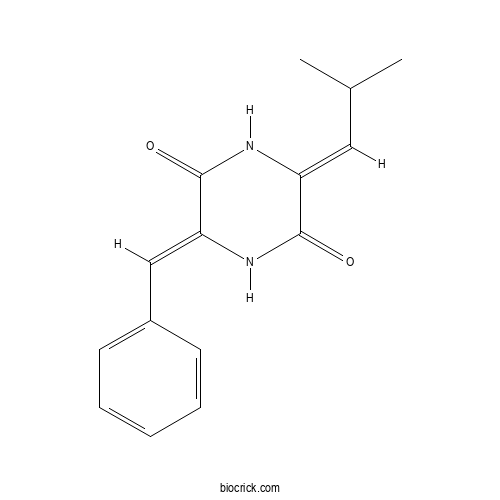
Chemical structure

3D structure
| Cas No. | 1222-90-8 | SDF | Download SDF |
| PubChem ID | 6109346 | Appearance | Powder |
| Formula | C15H16N2O2 | M.Wt | 256.30 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (3Z,6Z)-3-benzylidene-6-(2-methylpropylidene)piperazine-2,5-dione | ||
| SMILES | CC(C)C=C1C(=O)NC(=CC2=CC=CC=C2)C(=O)N1 | ||
| Standard InChIKey | LCIIOYPBHIZBOD-JMVBYTIWSA-N | ||
| Standard InChI | InChI=1S/C15H16N2O2/c1-10(2)8-12-14(18)17-13(15(19)16-12)9-11-6-4-3-5-7-11/h3-10H,1-2H3,(H,16,19)(H,17,18)/b12-8-,13-9- | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Albonoursin Dilution Calculator

Albonoursin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.9017 mL | 19.5084 mL | 39.0168 mL | 78.0336 mL | 97.5419 mL |
| 5 mM | 0.7803 mL | 3.9017 mL | 7.8034 mL | 15.6067 mL | 19.5084 mL |
| 10 mM | 0.3902 mL | 1.9508 mL | 3.9017 mL | 7.8034 mL | 9.7542 mL |
| 50 mM | 0.078 mL | 0.3902 mL | 0.7803 mL | 1.5607 mL | 1.9508 mL |
| 100 mM | 0.039 mL | 0.1951 mL | 0.3902 mL | 0.7803 mL | 0.9754 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 9''-O-Acetylsalcolin B
Catalog No.:BCX0554
CAS No.:910864-91-4
- Berchemol
Catalog No.:BCX0553
CAS No.:126882-59-5
- Daphnodorin H
Catalog No.:BCX0552
CAS No.:178897-27-3
- 1,5-Epoxy-3-hydroxy-1-(4-hydroxy-3,5-dimethoxyphenyl)-7-(4-hydroxy-3-methoxyphenyl)heptane
Catalog No.:BCX0551
CAS No.:719270-40-3
- meso-Octahydrocurcumin
Catalog No.:BCX0550
CAS No.:135413-63-7
- Daphnodorin G
Catalog No.:BCX0549
CAS No.:178664-65-8
- 9β-Hydroxynootkatone
Catalog No.:BCX0548
CAS No.:226547-01-9
- Isophthalic acid
Catalog No.:BCX0547
CAS No.:121-91-5
- (-)-Lyoniresinol 9-O-glucoside
Catalog No.:BCX0546
CAS No.:162613-63-0
- 1β-Hydroxy-12-noreremophila-6,9-diene-8,11-dione
Catalog No.:BCX0545
CAS No.:161127-52-2
- 9''-O-Acetylsalcolin A
Catalog No.:BCX0544
CAS No.:910864-92-5
- Sanggenol H
Catalog No.:BCX0543
CAS No.:202526-53-2
- 1,3-Di-O-caffeoyl-4-O-galloyl-β-D-glucopyranose
Catalog No.:BCX0556
CAS No.:359819-44-6
- 1-O-Caffeoyl-3,4,6-tri-O-galloyl-β-D-glucopyranose
Catalog No.:BCX0557
CAS No.:1219501-93-5
- Sanggenol I
Catalog No.:BCX0558
CAS No.:202526-54-3
- 3,4'-Dihydroxypropiophenone 3-O-glucoside
Catalog No.:BCX0559
CAS No.:53170-92-6
- α-Tocotrienol
Catalog No.:BCX0560
CAS No.:58864-81-6
- Phaeocaulisin F
Catalog No.:BCX0561
CAS No.:1422201-43-1
- 11-Hydroxyeremophil-1(10)-en-2-one
Catalog No.:BCX0562
CAS No.:20489-50-3
- Isoconiferinoside
Catalog No.:BCX0563
CAS No.:152686-86-7
- Trichilinin D
Catalog No.:BCX0564
CAS No.:220698-24-8
- Teucrenone
Catalog No.:BCX0565
CAS No.:152511-43-8
- 2,4,6,1'-Tetra-O-acetyl-3',6'-di-O-feruloylsucrose
Catalog No.:BCX0566
CAS No.:173614-59-0
- Alatusol D
Catalog No.:BCX0567
CAS No.:1578237-42-9
Cyclodipeptide oxidase is an enzyme filament.[Pubmed:37808672]
bioRxiv [Preprint]. 2023 Sep 25:2023.09.25.559410.
Modified cyclic dipeptides represent a widespread class of secondary metabolites with diverse pharmacological activities, including antibacterial, antifungal, and antitumor. Here, we report the structural characterization of the Streptomyces noursei enzyme AlbAB, a cyclodipeptide oxidase (CDO) carrying out alpha,beta-dehydrogenations during the biosynthesis of the antibiotic Albonoursin. We show that AlbAB is a megadalton heterooligomeric enzyme filament containing covalently bound flavin mononucleotide cofactors. We highlight that AlbAB filaments consist of alternating dimers of AlbA and AlbB and that enzyme activity is crucially dependent on filament formation. We show that AlbA-AlbB interactions are highly conserved suggesting that all CDO-like enzymes are likely enzyme filaments. Our work represents the first structural characterization of a CDO. As CDOs have been employed in the structural diversification of cyclic dipeptides, our results will be useful for future applications of CDOs in biocatalysis and chemoenzymatic synthesis.
Identification of Effective and Nonpromiscuous Antidiabetic Drug Molecules from Penicillium Species.[Pubmed:35722152]
Evid Based Complement Alternat Med. 2022 Jun 8;2022:7040547.
Diabetes mellitus (DM) is a very common metabolic disorder/disease. The deterioration of beta-cells by autoimmune system is the hallmark of this disease. Thioredoxin-Interacting Protein (TXNIP) is responsible for beta-cells degradation by T-cells in the pancreas. This protein had been declared a good drug target for controlling DM. Lots of side effects have been reported as a result of long-time consumption of conventional antidiabetic drugs. The development of new and effective drugs with the minimal side effects needs time. TXNIP was selected as a target for Computer-Aided Drug Design. The antidiabetic fungal metabolite compounds were selected from the literature. The compounds were screened for their drug-likeness properties by DruLiTo and DataWarior tools. Twenty-two drug-like fungal compounds were subjected to Quantitative Structure-Activity Relationship (QSAR) analysis by using CheS-Mapper 2.0. The lowest (0.01) activity cliff was found for three compounds: Pinazaphilone A, Pinazaphilone B, and Chermesinone A. The highest value for apol (81.76) was shown by Asperphenamate, while Albonoursin and Sterenin L showed highest score (40.66) for bpol. The lowest value (0.46) for fractional molecular frame (FMF) was calculated for Pinazaphilone A and Pinazaphilone B. TPSA for Pinazaphilone A and Pinazaphilone B was 130.51 A(2). log P < 5 was observed for all the twenty-two compounds. Molecular docking of fungal compounds with TXNIP was done by AutoDock Vina. The binding energy for complexes ranged between -9.2 and -4.6 kcal/mol. Four complexes, TXNIP-Pinazaphilone A, TXNIP-Pinazaphilone B, TXNIP-Asperphenamate, and TXNIP-Sterenin L, were selected for MD simulation to find out the best lead molecule. Only one complex, TXNIP-Pinazaphilone B, showed a stable conformation throughout the 80 ns run of MD simulation. Pinazaphilone B derived from the Penicillium species fungi was selected as the lead molecule for development of antidiabetic drug having the least side effects.
Modular and Integrative Vectors for Synthetic Biology Applications in Streptomyces spp.[Pubmed:31175189]
Appl Environ Microbiol. 2019 Aug 1;85(16):e00485-19.
With the development of synthetic biology in the field of (actinobacterial) specialized metabolism, new tools are needed for the design or refactoring of biosynthetic gene clusters. If libraries of synthetic parts (such as promoters or ribosome binding sites) and DNA cloning methods have been developed, to our knowledge, not many vectors designed for the flexible cloning of biosynthetic gene clusters have been constructed. We report here the construction of a set of 12 standardized and modular vectors designed to afford the construction or the refactoring of biosynthetic gene clusters in Streptomyces species, using a large panel of cloning methods. Three different resistance cassettes and four orthogonal integration systems are proposed. In addition, FLP recombination target sites were incorporated to allow the recycling of antibiotic markers and to limit the risks of unwanted homologous recombination in Streptomyces strains when several vectors are used. The functionality and proper integration of the vectors in three commonly used Streptomyces strains, as well as the functionality of the Flp-catalyzed excision, were all confirmed. To illustrate some possible uses of our vectors, we refactored the Albonoursin gene cluster from Streptomyces noursei using the BioBrick assembly method. We also used the seamless ligase chain reaction cloning method to assemble a transcription unit in one of the vectors and genetically complement a mutant strain.IMPORTANCE One of the strategies employed today to obtain new bioactive molecules with potential applications for human health (for example, antimicrobial or anticancer agents) is synthetic biology. Synthetic biology is used to biosynthesize new unnatural specialized metabolites or to force the expression of otherwise silent natural biosynthetic gene clusters. To assist the development of synthetic biology in the field of specialized metabolism, we constructed and are offering to the community a set of vectors that were intended to facilitate DNA assembly and integration in actinobacterial chromosomes. These vectors are compatible with various DNA cloning and assembling methods. They are standardized and modular, allowing the easy exchange of a module by another one of the same nature. Although designed for the assembly or the refactoring of specialized metabolite gene clusters, they have a broader potential utility, for example, for protein production or genetic complementation.
Two New Piperazine-Triones from a Marine-Derived Streptomycetes sp. Strain SMS636.[Pubmed:30901830]
Mar Drugs. 2019 Mar 21;17(3):186.
Two new piperazine-triones lansai E and F (1, 2), together with four known secondary metabolites lansai D (3), 1-N-methyl-(E,Z)-Albonoursin (4), imidazo[4,5-e]-1,2,4-triazine (5), and streptonigrin (6) were isolated from a deep-sea-derived Streptomycetes sp. strain SMS636. The structures of the isolated compounds were confirmed by comprehensive spectroscopic analysis, including HRESIMS, 1D and 2D NMR. Compound 4 exhibited moderate antibacterial activities against Staphylococcus aureus and methicillin resistant S. aureus (MRSA) with Minimum Inhibitory Concentration (MIC) values of 12.5 and 25 mug/mL, respectively. Compound 6 displayed significant antibacterial activities against S. aureus, MRSA and Bacillus Calmette-Guerin (BCG) with MIC values of 0.78, 0.78 and 1.25 mug/mL, respectively.
Analysis of the biosynthesis of antibacterial cyclic dipeptides in Nocardiopsis alba.[Pubmed:25048158]
Arch Microbiol. 2014 Nov;196(11):765-74.
Nocardiopsis alba is frequently isolated from environment and has recently been suggested as a casual symbiotic actinobacterium of diverse invertebrates. Using activity-guided fractionation, we purified two antibacterial cyclic dipeptides, cyclo(DeltaPhe-DeltaLeu) (Albonoursin) and cyclo(DeltamTyr-DeltaLeu), from a culture of Nocardiopsis alba ATCC BAA-2165. Analysis of N. alba genome revealed genetic information similar to Albonoursin biosynthetic gene cluster, albABC. An albABC gene deletion mutant of N. alba was generated. Liquid chromatography-mass spectrometry analysis showed that the mutant could not produce the cyclic dipeptides. Cyclic dipeptide production in the mutant was restored by genetic complementation with the albABC cloned in a native plasmid of Nocardiopsis. beta-Glucuronidase reporter assays with a second mutant construct, in which albABC promoter is transcriptionally fused to the reporting gene gusA, indicated that albABC gene expression was subject to osmoregulation. The system presented will be used to study the metabolic and genetic control of cyclic dipeptide biosynthesis in Nocardiopsis.
Cyclodipeptide synthases are a family of tRNA-dependent peptide bond-forming enzymes.[Pubmed:19430487]
Nat Chem Biol. 2009 Jun;5(6):414-20.
Cyclodipeptides and their derivatives belong to the diketopiperazine (DKP) family, which is comprised of a broad array of natural products that exhibit useful biological properties. In the few known DKP biosynthetic pathways, nonribosomal peptide synthetases (NRPSs) are involved in the synthesis of cyclodipeptides that constitute the DKP scaffold, except in the Albonoursin (1) pathway. Albonoursin, or cyclo(alpha,beta-dehydroPhe-alpha,beta-dehydroLeu), is an antibacterial DKP produced by Streptomyces noursei. In this pathway, the formation of the cyclo(Phe-Leu) (2) intermediate is catalyzed by AlbC, a small protein unrelated to NRPSs. We demonstrated that AlbC uses aminoacyl-tRNAs as substrates to catalyze the formation of the DKP peptide bonds. Moreover, several other bacterial proteins, presenting moderate similarity to AlbC, also use aminoacyl-tRNAs to synthesize various cyclodipeptides. Therefore, AlbC and these related proteins belong to a newly defined family of enzymes that we have named cyclodipeptide synthases (CDPSs).
Enzymatic conversion of cyclic dipeptides to dehydro derivatives that inhibit cell division.[Pubmed:16232823]
J Biosci Bioeng. 2000;90(1):86-9.
The cell-free extract of an Albonoursin-producing strain, Streptomyces albulus KO-23, was found to catalyze the conversion of several cyclic dipeptides having Phe and aliphatic side chain-containing amino acid residues to the corresponding dehydro derivatives. 3Z-Benzylidene-6S-methyl-2,5-piperazinedione, 3Z-benzylidene-2,5-piperazinedione, and 3Z, 6Z-dibenzylidene-2,5-piperazinedione were prepared by this conversion system. Among the dehydro cyclic dipeptides prepared, tetradehydro derivatives exhibited inhibitory activity toward the first cleavage of sea urchin embryo, while didehydro derivatives did not. We previously found that cyclo(Leu-Phe) and its didehydro derivatives did not show any inhibitory activity, in contrast to high activity in the case of Albonoursin. Taken together, these findings indicate that dehydrogenation at the alpha,beta-positions of both amino acid residues in this type of cyclic dipeptide is required for the inhibitory activity.
Enzymatic synthesis of dehydro cyclo(His-Phe)s, analogs of the potent cell cycle inhibitor, dehydrophenylahistin, and their inhibitory activities toward cell division.[Pubmed:15564674]
Biosci Biotechnol Biochem. 2004 Nov;68(11):2341-5.
Cyclo(His-Phe) was effectively converted to its dehydro derivatives by the enzyme of Streptomyces albulus KO-23, an Albonoursin-producing actinomycete. Two types of dehydro derivatives were isolated from the reaction mixture and identified as cyclo(DeltaHis-DeltaPhe) and cyclo(His-DeltaPhe). This is the first report on cyclo(His-DeltaPhe) and the enzymatic preparation of both compounds. Cyclo(DeltaHis-DeltaPhe), a tetradehydro cyclic dipeptide, exhibited a minimum inhibitory concentration of 0.78 mumol/ml inhibitory activity toward the first cleavage of sea urchin embryos, in contrast to cyclo(His-DeltaPhe) that had no activity. The finding that the isoprenylated derivative of cyclo(DeltaHis-DeltaPhe), dehydrophyenylahistin, had 2,000 times higher activity than cyclo(DeltaHis-DeltaPhe) indicates that an isoprenyl group attached to an imidazole ring of the compound was essential for the inhibitory activity.
A novel potent cell cycle inhibitor dehydrophenylahistin--enzymatic synthesis and inhibitory activity toward sea urchin embryo.[Pubmed:12617513]
J Antibiot (Tokyo). 2002 Dec;55(12):1042-7.
A novel dehydrogenated cyclic dipeptide named as dehydrophenylahistin (deltaPLH) was effectively prepared from a fungal metabolite (-)-phenylahistin by the enzymatic conversion catalyzed by the cell-free extract of Streptomyces albulus KO-23, an Albonoursin-producing actinomycete. deltaPLH exhibited more than 1,000 times as high potent inhibitory activity toward the first cleavage of sea urchin embryos as (-)-phenylahisitn which has been reported to be a cell cycle inhibitor and more than 10,000 as high as Albonoursin, indicating that deltaPLH is a promising leading compound for anticancer drugs.
The albonoursin gene Cluster of S noursei biosynthesis of diketopiperazine metabolites independent of nonribosomal peptide synthetases.[Pubmed:12498889]
Chem Biol. 2002 Dec;9(12):1355-64.
Albonoursin [cyclo(deltaPhe-DeltaLeu)], an antibacterial peptide produced by Streptomyces noursei, is one of the simplest representatives of the large diketopiperazine (DKP) family. Formation of alpha,beta unsaturations was previously shown to occur on cyclo(L-Phe-L-Leu), catalyzed by the cyclic dipeptide oxidase (CDO). We used CDO peptide sequence information to isolate a 3.8 kb S. noursei DNA fragment that directs Albonoursin biosynthesis in Streptomyces lividans. This fragment encompasses four complete genes: albA and albB, necessary for CDO activity; albC, sufficient for cyclic dipeptide precursor formation, although displaying no similarity to non ribosomal peptide synthetase (NRPS) genes; and albD, encoding a putative membrane protein. This first isolated DKP biosynthetic gene cluster should help to elucidate the mechanism of DKP formation, totally independent of NRPS, and to characterize novel DKP biosynthetic pathways that could be engineered to increase the molecular diversity of DKP derivatives.
Cyclic dipeptide oxidase from Streptomyces noursei. Isolation, purification and partial characterization of a novel, amino acyl alpha,beta-dehydrogenase.[Pubmed:11248691]
Eur J Biochem. 2001 Mar;268(6):1712-21.
Cyclic dipeptide oxidase is a novel enzyme that specifically catalyzes the formation of alpha,beta-dehydro-Phe (Delta Phe) and alpha,beta-dehydro-Leu (Delta Leu) residues during the biosynthesis of Albonoursin, cyclo(Delta Phe-Delta Leu), an antibiotic produced by Streptomyces noursei. It was purified 600-fold with a 30% overall recovery, and consists of the association of a single type of subunit with a relative molecular mass of 21,066 resulting in a large homopolymer of relative molecular mass over 2,000,000. The enzyme exhibits a typical flavoprotein spectrum with maxima at 343.5 and 447.5 nm, the flavin prosthetic group being covalently bound to the protein. The catalytic reaction of the natural substrate cyclo(L-Phe-L-Leu) occurs in a two-step sequential reaction leading first to cyclo(alpha,beta-dehydro-Phe-L-Leu) and finally to Albonoursin. Kinetic parameters for the first step were determined (K(m) = 53 microM; k = 0.69 s(-1)). The enzyme was shown to catalyze the conversion of a variety of cyclo(dipeptides) and can be reoxidized at the expense of molecular oxygen by producing H(2)O(2). This reaction mechanism, which differs from those already described for the formation of alpha,beta-dehydro-amino acids, might consist of the transient formation of an intermediate imine followed by its rearrangement into an alpha,beta-dehydro-residue.
Biosynthetic intermediates of the tetradehydro cyclic dipeptide albonoursin produced by Streptomyces albulus KO-23.[Pubmed:11213286]
J Antibiot (Tokyo). 2000 Nov;53(11):1257-64.
The cell-free extract of an Albonoursin-producing strain Streptomyces albulus KO-23 catalyzes the conversion of cyclo(L-Leu-L-Phe) (1) to Albonoursin (2). At the early stage of this conversion, two compounds were newly formed prior to Albonoursin synthesis in the reaction mixture. These compounds were isolated and identified as (Z)-3-benzylidene-6-isobutyl-2,5-piperazinedione (4) and (Z)-3-benzyl-6-isobutylidene-2,5-piperazinedione (3). The cell-free extract also catalyzed the conversion of compound 3 or 4 to Albonoursin. From these results, Albonoursin was found to be biosynthesized via these compounds from cyclo(L-Leu-L-Phe). These didehydro diketopiperazines exhibited no inhibitory activity toward the first cleavage of sea urchin embryo in contrast to the higher cytotoxicity for Albonoursin, indicating that dehydrogenation at alpha,beta-positions of both amino acid residues in diketopiperazines is required for cytotoxicity.
Effective production of dehydro cyclic dipeptide albonoursin exhibiting pronuclear fusion inhibitory activity. II. Biosynthetic and bioconversion studies.[Pubmed:10724009]
J Antibiot (Tokyo). 2000 Jan;53(1):58-62.
Albonoursin production was greatly enhanced when cyclo (L-Leu-L-Phe) (CFL), a tetrahydro derivative of Albonoursin, was added to the 2-day culture of an Albonoursin-producing actinomycete, Streptomyces albulus KO-23. The increase in Albonoursin production paralleled the amount of CFL added. Furthermore, the resting cells of the strain catalyzed the bioconversion of CFL to Albonoursin. The optimum pH and temperature for the conversion were found to be pH 10.0 and 50 degrees C. The feeding experiments and the resting-cell reactions revealed that Albonoursin is biosynthesized by dehydrogenation of CFL in the actinomycete. This is the first report for a dehydrogenation of amino acid residues at the alpha,beta-positions in cyclic dipeptides.
Effective production of dehydro cyclic dipeptide albonoursin exhibiting pronuclear fusion inhibitory activity. I. Taxonomy and fermentation.[Pubmed:10656574]
J Antibiot (Tokyo). 1999 Nov;52(11):1017-22.
Strain KO-23, an actinomycete producing Albonoursin as well as streptopyrone, was identified as Streptomyces albulus by morphological and biochemical studies. Fermentation conditions for Albonoursin, a dehydro cyclic dipeptide exhibiting a pronounced inhibitory activity toward pronuclear fusion of sea urchin eggs, were optimized. Under the optimum conditions, the actinomycete produced 16 mg/liter of Albonoursin, 30 times higher than that in the original culture. The cells cultivated under these conditions highly express biosynthetic enzymes for Albonoursin, and thus are available for biosynthetic studies of dehydro cyclic peptides.


