AcrovestoneCAS# 24177-16-0 |
Quality Control & MSDS
Number of papers citing our products

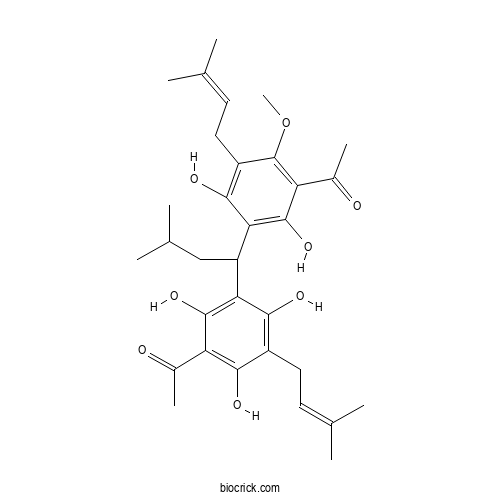
Chemical structure

3D structure
| Cas No. | 24177-16-0 | SDF | Download SDF |
| PubChem ID | 159969 | Appearance | Powder |
| Formula | C32H42O8 | M.Wt | 554.7 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 1-[3-[1-[3-acetyl-2,6-dihydroxy-4-methoxy-5-(3-methylbut-2-enyl)phenyl]-3-methylbutyl]-2,4,6-trihydroxy-5-(3-methylbut-2-enyl)phenyl]ethanone | ||
| SMILES | CC(C)CC(C1=C(C(=C(C(=C1O)CC=C(C)C)O)C(=O)C)O)C2=C(C(=C(C(=C2O)CC=C(C)C)OC)C(=O)C)O | ||
| Standard InChIKey | KLFWXYAHGSXKAW-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C32H42O8/c1-15(2)10-12-20-27(35)23(18(7)33)30(38)25(28(20)36)22(14-17(5)6)26-29(37)21(13-11-16(3)4)32(40-9)24(19(8)34)31(26)39/h10-11,17,22,35-39H,12-14H2,1-9H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Acrovestone Dilution Calculator

Acrovestone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.8028 mL | 9.0139 mL | 18.0278 mL | 36.0555 mL | 45.0694 mL |
| 5 mM | 0.3606 mL | 1.8028 mL | 3.6056 mL | 7.2111 mL | 9.0139 mL |
| 10 mM | 0.1803 mL | 0.9014 mL | 1.8028 mL | 3.6056 mL | 4.5069 mL |
| 50 mM | 0.0361 mL | 0.1803 mL | 0.3606 mL | 0.7211 mL | 0.9014 mL |
| 100 mM | 0.018 mL | 0.0901 mL | 0.1803 mL | 0.3606 mL | 0.4507 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- [6]-Gingerol
Catalog No.:BCN0483
CAS No.:39886-76-5
- Stigmalactam
Catalog No.:BCN0482
CAS No.:289499-72-5
- Isorengyol
Catalog No.:BCN0481
CAS No.:101489-38-7
- 4-(Acetoxymethyl)phenyl glucoside
Catalog No.:BCN0480
CAS No.:188290-72-4
- Kokusaginine
Catalog No.:BCN0479
CAS No.:484-08-2
- 2,4,6-Trihydroxy-3-prenylacetophenone
Catalog No.:BCN0478
CAS No.:27364-71-2
- Ferruginoside C
Catalog No.:BCN0477
CAS No.:213991-03-8
- (±)-8-Gingerol
Catalog No.:BCN0476
CAS No.:77398-92-6
- 2',3'-Dehydromarmesin
Catalog No.:BCN0475
CAS No.:28664-60-0
- Polypodine B 20,22-acetonide
Catalog No.:BCN0474
CAS No.:159858-85-2
- Acrotrione
Catalog No.:BCN0473
CAS No.:2349327-31-5
- 1,4-Dihydro-1-methyl-4-oxonicotinamide
Catalog No.:BCN0472
CAS No.:769-49-3
- Marionol
Catalog No.:BCN0485
CAS No.:65602-55-3
- Verproside
Catalog No.:BCN0486
CAS No.:50932-20-2
- 5-Hydroxy-6,7,3',4'-tetramethoxyflavone
Catalog No.:BCN0487
CAS No.:21763-80-4
- Piperdardine
Catalog No.:BCN0488
CAS No.:188426-70-2
- 4''-O-Methylcatalposide
Catalog No.:BCN0489
CAS No.:887140-17-2
- Apigenin 7,4'-di-O-alloside
Catalog No.:BCN0490
CAS No.:95693-63-3
- Tsaokoin
Catalog No.:BCN0491
CAS No.:343605-41-4
- Piperchabamide B
Catalog No.:BCN0492
CAS No.:807618-21-9
- Piceatannol 4'-O-glucoside
Catalog No.:BCN0493
CAS No.:116181-54-5
- 6-O-Veratroylcatalpol
Catalog No.:BCN0494
CAS No.:56973-43-4
- Uvamalol D
Catalog No.:BCN0495
CAS No.:545404-02-2
- Muramine
Catalog No.:BCN0496
CAS No.:2292-20-8
Acroflavone A, a new prenylated flavone from the fruit of Acronychia pedunculata (L.) Miq.[Pubmed:34126824]
Nat Prod Res. 2021 Jun 15:1-7.
Phytochemical investigation of the fruit of Acronychia pedunculata (L.) Miq. led to the isolation of a new prenylated flavone, acroflavone A (1), together with eight known compounds (2-9). Their structures were elucidated by thorough analysis of mass spectrometric and NMR spectroscopic data. The isolated compounds were evaluated against several bacterial strains. Three known compounds (3-5) demonstrated antibacterial activities. Among them, Acrovestone (5) proved equally or more potent than chloramphenicol against three out of the four strains tested.
Prenylated Acetophloroglucinol Dimers from Acronychia trifoliolata: Structure Elucidation and Total Synthesis.[Pubmed:31550158]
J Nat Prod. 2019 Oct 25;82(10):2852-2858.
The isolation of 12 secondary metabolites, including seven new acetophenone monomers, from the 50% CH3OH/CH2Cl2 extract (N089419-L/6) of Acronychia trifoliolata was reported previously. In the present work, three new prenylated acetophenone dimers (1-3) and five known dimers (4-8) were isolated, and their structures were elucidated by using various NMR spectroscopic techniques and HRMS. Among the new dimers, an unprecedented 4-isobutyl-3-isopropyltetrahydro-2H-pyran ring was observed in the structure of 1. This study is the first to report the formation of a 2H-pyran ring between two prenylated acetophloroglucinols. Only four related dimers have been reported before, and they were formylated phloroglucinol dimers from the family Eucalypteae. Compounds 2 and 3 are Acrovestone-like dimers, and the structure of 3 was confirmed by total synthesis. The evaluation of the antiproliferative activity of isolated and synthesized Acrovestone-like dimers indicated that a double bond in the prenyl-like moiety as found in the more active compounds might be important for mediating activity, while the pendant isobutyl group seems to be less important.
Acetophenones Isolated from Acronychia pedunculata and their Anti-proliferative Activities.[Pubmed:26996027]
Nat Prod Commun. 2016 Jan;11(1):83-6.
Study of the chemical constituents of Acronychia pedunculata (L.) Miq. (Rutaceae) stems collected in Taiwan led to the isolation and identification of eight known and three new acetophenones, named acrophenone A (1), B (2), and C (3). Of them, Acrovestone (5), acropyrone (6) and acrovestenol (7), which are dimer compounds, strikingly inhibited the proliferation of human leukemia cell lines.
Cytotoxic prenylated acetophenone dimers from Acronychia pedunculata.[Pubmed:22708987]
J Nat Prod. 2012 Jul 27;75(7):1270-6.
Three new acetophenone dimers or Acronychia-type acetophenones, acropyrone (1), acropyranol A (2), and acropyranol B (3), were isolated from the trunk bark of Acronychia pedunculata and structurally characterized, together with four known acetophenone dimers, Acrovestone (4), acrovestenol (5), acrofolione A (6), and acrofolione B (7), the acetophenone monomer acronyline (8), and four furoquinoline alkaloids. The chemical structures of the new isolated compounds were elucidated unambiguously by spectroscopic data analysis. The cytotoxic activities of the isolated acetophenone dimers were evaluated against the DU145 prostate and A2058 melanoma human cancer cell lines as well as the NHDF normal cell line. Acrovestone (4) and acrovestenol (5) exhibited substantial cytotoxicity, with IC(50) values of 0.38 and 2.8 muM against A2058 melanoma cells as well as 0.93 and 2.7 muM against DU145 prostate cancer cells, respectively.
Acetophenone derivatives from Acronychia pedunculata.[Pubmed:12880321]
J Nat Prod. 2003 Jul;66(7):990-3.
Chemical investigation on the stem and root bark of Acronychia pedunculata has resulted in the isolation of five new acetophenones, namely, acronyculatins A (1), B (2), C (3), D (4), and E (5). The structures of these metabolites were established on the basis of their 1D and 2D NMR spectroscopic and mass spectrometric data and by CD spectroscopy. The antioxidant and antityrosinase activities of these five metabolites and Acrovestone (6) were evaluated. Among these compounds, 6 showed marginal antioxidant and antityrosinase activities.
X-ray crystal structure of acrovestone, a cytotoxic principle from Acronychia pedunculata.[Pubmed:2614422]
J Nat Prod. 1989 Nov-Dec;52(6):1284-9.
Acrovestone was isolated from the stem and root back of Acronychia pedunculata and shown for the first time to be a cytotoxic principle. Its structure, derived from spectral data, was completely characterized by single-crystal X-ray analysis.
[Studies on the chemical constituents of Acronychia pedunculata (L.) Mig].[Pubmed:2506892]
Zhongguo Zhong Yao Za Zhi. 1989 Feb;14(2):30-1, 62.
Two crystalline substances were isolated from the stem bark of Acronychia pedunculata and identified as Acrovestone and bauerenol on the basis of chemical studies and spectrometric analysis.


