Acerogenin GCAS# 130233-83-9 |
Quality Control & MSDS
Number of papers citing our products

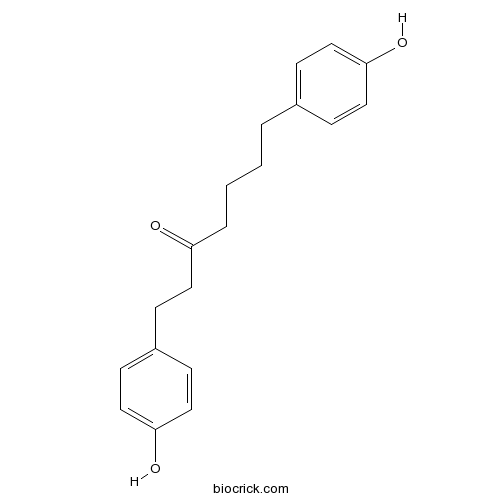
Chemical structure

3D structure
| Cas No. | 130233-83-9 | SDF | Download SDF |
| PubChem ID | 14608480 | Appearance | Oil |
| Formula | C19H22O3 | M.Wt | 298.38 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 1,7-bis(4-hydroxyphenyl)heptan-3-one | ||
| SMILES | C1=CC(=CC=C1CCCCC(=O)CCC2=CC=C(C=C2)O)O | ||
| Standard InChIKey | QUHYUSAHBDACNG-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C19H22O3/c20-17(10-7-16-8-13-19(22)14-9-16)4-2-1-3-15-5-11-18(21)12-6-15/h5-6,8-9,11-14,21-22H,1-4,7,10H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Acerogenin G is a natural product from Acer nikoense. |
| Structure Identification | Tetrahedron, 2013, 69(13):2807-2815.Total synthesis of acerogenins E, G and K, and centrolobol.[Reference: WebLink]
|

Acerogenin G Dilution Calculator

Acerogenin G Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.3514 mL | 16.7572 mL | 33.5143 mL | 67.0286 mL | 83.7858 mL |
| 5 mM | 0.6703 mL | 3.3514 mL | 6.7029 mL | 13.4057 mL | 16.7572 mL |
| 10 mM | 0.3351 mL | 1.6757 mL | 3.3514 mL | 6.7029 mL | 8.3786 mL |
| 50 mM | 0.067 mL | 0.3351 mL | 0.6703 mL | 1.3406 mL | 1.6757 mL |
| 100 mM | 0.0335 mL | 0.1676 mL | 0.3351 mL | 0.6703 mL | 0.8379 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- N-(4-Hydroxyphenylacetyl)spermine
Catalog No.:BCC6594
CAS No.:130210-32-1
- Cirsimarin
Catalog No.:BCN6821
CAS No.:13020-19-4
- Torilin
Catalog No.:BCN6611
CAS No.:13018-10-5
- Torilolone
Catalog No.:BCN6659
CAS No.:13018-09-2
- Ergosterol glucoside
Catalog No.:BCN7327
CAS No.:130155-33-8
- (+)-Igmesine hydrochloride
Catalog No.:BCC5902
CAS No.:130152-35-1
- Lomustine
Catalog No.:BCC4794
CAS No.:13010-47-4
- Mafenide Acetate
Catalog No.:BCC5236
CAS No.:13009-99-9
- Dehydrocorydaline nitrate
Catalog No.:BCN2745
CAS No.:13005-09-9
- GSK1324726A
Catalog No.:BCC4038
CAS No.:1300031-52-0
- I-BET151 (GSK1210151A)
Catalog No.:BCC4476
CAS No.:1300031-49-5
- Quinine
Catalog No.:BCN2341
CAS No.:130-95-0
- 3'-Demethoxypiplartine
Catalog No.:BCN4021
CAS No.:130263-10-4
- PD 135158
Catalog No.:BCC7431
CAS No.:130285-87-9
- Rubiarbonol B
Catalog No.:BCN6159
CAS No.:130288-60-7
- Fmoc-Asp(OcHex)-OH
Catalog No.:BCC3468
CAS No.:130304-80-2
- HOE 140
Catalog No.:BCC5964
CAS No.:130308-48-4
- Fmoc-D-Tic-OH
Catalog No.:BCC3342
CAS No.:130309-33-0
- Fmoc-Oic-OH
Catalog No.:BCC3305
CAS No.:130309-37-4
- H-D-Phe-OMe.HCl
Catalog No.:BCC3013
CAS No.:13033-84-6
- CI 988
Catalog No.:BCC7430
CAS No.:130332-27-3
- Cathayanon H
Catalog No.:BCN3570
CAS No.:1303438-51-8
- Cathayanon I
Catalog No.:BCN3678
CAS No.:1303438-52-9
- MI-773
Catalog No.:BCC5155
CAS No.:1303607-07-9
Total synthesis of acerogenins E, G and K, and centrolobol.
Tetrahedron, 2013, 69(13):2807-2815.
The first total synthesis of the diarylheptanoid acerogenins E and K, isolated from Acer nikoense MAXIM., is described. Formation of the 13-membered m,m-cyclophane skeleton was successfully achieved on the basis of a domino process involving a Miyaura arylborylation–intramolecular Suzuki reaction. The cyclization precursor was prepared via a Wittig reaction and Claisen–Schmidt condensation, which proceeded in moderate yields. The total synthesis of Acerogenin G and centrolobol was also achieved from a common synthetic intermediate.


