AR-C 102222Selective iNOS inhibitor CAS# 253771-21-0 |
- TDZD-8
Catalog No.:BCC4258
CAS No.:327036-89-5
- TWS119
Catalog No.:BCC4512
CAS No.:601514-19-6
- GSK-3 inhibitor 1
Catalog No.:BCC4126
CAS No.:603272-51-1
- LY2090314
Catalog No.:BCC1717
CAS No.:603288-22-8
- 1-Azakenpaullone
Catalog No.:BCC5332
CAS No.:676596-65-9
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 253771-21-0 | SDF | Download SDF |
| PubChem ID | 21192822 | Appearance | Powder |
| Formula | C19H17ClF2N6O | M.Wt | 418.83 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 25 mM in water and to 100 mM in DMSO | ||
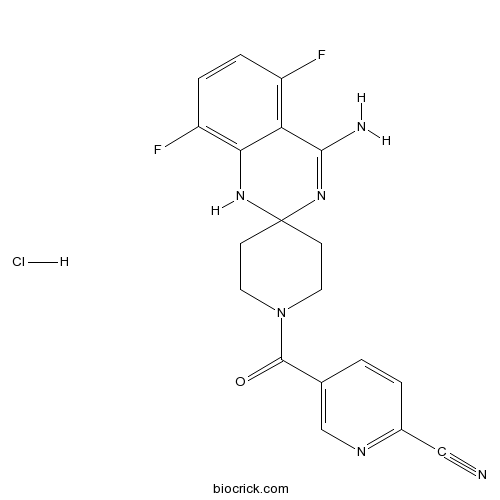
| Chemical Name | 5-(4-amino-5,8-difluorospiro[1H-quinazoline-2,4'-piperidine]-1'-carbonyl)pyridine-2-carbonitrile;hydrochloride | ||
| SMILES | C1CN(CCC12NC3=C(C=CC(=C3C(=N2)N)F)F)C(=O)C4=CN=C(C=C4)C#N.Cl | ||
| Standard InChIKey | FJFMOBYDHGJDGV-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C19H16F2N6O.ClH/c20-13-3-4-14(21)16-15(13)17(23)26-19(25-16)5-7-27(8-6-19)18(28)11-1-2-12(9-22)24-10-11;/h1-4,10,25H,5-8H2,(H2,23,26);1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Inducible nitric oxide synthase (iNOS) inhibitor; selective for iNOS over eNOS (IC50 values are 0.037 and >100 μM for iNOS and eNOS respectively). Exhibits antinociceptive and anti-inflammatory activity in rodent pain models. |

AR-C 102222 Dilution Calculator

AR-C 102222 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3876 mL | 11.938 mL | 23.876 mL | 47.7521 mL | 59.6901 mL |
| 5 mM | 0.4775 mL | 2.3876 mL | 4.7752 mL | 9.5504 mL | 11.938 mL |
| 10 mM | 0.2388 mL | 1.1938 mL | 2.3876 mL | 4.7752 mL | 5.969 mL |
| 50 mM | 0.0478 mL | 0.2388 mL | 0.4775 mL | 0.955 mL | 1.1938 mL |
| 100 mM | 0.0239 mL | 0.1194 mL | 0.2388 mL | 0.4775 mL | 0.5969 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- TRIM
Catalog No.:BCC6847
CAS No.:25371-96-4
- Asperulosidic acid
Catalog No.:BCN3088
CAS No.:25368-11-0
- Litseglutine B
Catalog No.:BCN5120
CAS No.:25368-01-8
- 2-Amino-6-methylbenzothiazole
Catalog No.:BCC8543
CAS No.:2536-91-6
- 16-O-Acetylpolyporenic acid C
Catalog No.:BCN4058
CAS No.:2535-06-0
- Wiskostatin
Catalog No.:BCC7934
CAS No.:253449-04-6
- (+)-Corlumidine
Catalog No.:BCN2662
CAS No.:25344-54-1
- Viniferin
Catalog No.:BCN3757
CAS No.:253435-07-3
- Trazodone HCl
Catalog No.:BCC5032
CAS No.:25332-39-2
- BMS 195614
Catalog No.:BCC7740
CAS No.:253310-42-8
- Isocalamendiol
Catalog No.:BCN5119
CAS No.:25330-21-6
- Tetrabromobisphenol A diallyl ether
Catalog No.:BCC9174
CAS No.:25327-89-3
- Kanamycin Sulfate
Catalog No.:BCC1205
CAS No.:25389-94-0
- Morroniside
Catalog No.:BCN5009
CAS No.:25406-64-8
- Boc-ß-HoAsp(OBzl)-OH
Catalog No.:BCC3229
CAS No.:254101-10-5
- Phellopterin
Catalog No.:BCN2637
CAS No.:2543-94-4
- H-Cys(pMeOBzl)-OH
Catalog No.:BCC2909
CAS No.:2544-31-2
- Dehydropitavastatin ethyl ester
Catalog No.:BCC8931
CAS No.:254452-91-0
- (+)-Afzelechin
Catalog No.:BCN5121
CAS No.:2545-00-8
- Felbamate
Catalog No.:BCC4904
CAS No.:25451-15-4
- H-D-Glu-OtBu
Catalog No.:BCC2938
CAS No.:25456-76-2
- H-Asn-OtBu
Catalog No.:BCC2877
CAS No.:25456-86-4
- Demethyleneberberine
Catalog No.:BCN2829
CAS No.:25459-91-0
- 2-MPPA
Catalog No.:BCC7995
CAS No.:254737-29-6
Microwave spectroscopic and atoms in molecules theoretical investigations on the Ar...propargyl alcohol complex: Ar...H-O, Ar...pi, and Ar...C interactions.[Pubmed:23292768]
Chemphyschem. 2013 Mar 18;14(4):754-63.
The structure of the Ar...propargyl alcohol (Ar...PA) complex is determined from the rotational spectra of the parent complex and its two deuterated isotopologues, namely Ar...PA-D(OD) and Ar...PA-D(CD). The spectra confirm a geometry in which PA exists in the gauche form with Ar located in between -OH and -C identical withC-H groups. All a, b and c types of transitions show small splitting due to some large-amplitude motion dominated by C-OH torsion, as in the monomer. Splittings in a- and b-type transitions are of the order of a few kilohertz, whereas splitting in the c-type transitions is relatively larger (0.9-2.6 MHz) and decreases in the order Ar...PA>Ar...PA-D(CD)>Ar...PA-D(OD). The assignments are well supported by ab initio calculations. Atoms in molecules (AIM) and electrostatic potential calculations are used to explore the nature of the interactions in this complex. AIM calculations not only reveal the expected O-H...Ar and pi...Ar interactions in the Ar...gauche-PA complex, but also novel C...Ar (of CH2OH group) and O-H...Ar interactions in the Ar...trans-PA complex. Similar interactions are also present in the Ar...methanol complex.
Bond activation with an apparently benign ethynyl dithiocarbamate Ar-C identical withC-S-C(S)NR2.[Pubmed:23210141]
Angew Chem Int Ed Engl. 2011 Oct 10;50(42):9923-5.
The hedgehog molecule: A simple ethynyl dithiocarbamate [Ar-C identical withC-S-C(S)NR(2)] is able to cleave a broad range of enthalpically strong sigma bonds and to activate carbon dioxide and elemental sulfur. Depending on the substrate, the bond activation process involves either the existence of an equilibrium with the nonobservable mesoionic carbene isomer or the cooperation of the nucleophilic carbon-carbon triple bond and the electrophilic CS carbon atom.
Infiltrating neutrophils promote renal cell carcinoma (RCC) proliferation via modulating androgen receptor (AR) --> c-Myc signals.[Pubmed:26231735]
Cancer Lett. 2015 Nov 1;368(1):71-78.
Early studies found critical roles for neutrophils in renal cell carcinoma (RCC) progression. However, detailed mechanisms of how infiltrating neutrophils in the kidney tumor microenvironment impact RCC progression remain unclear. Here we found more neutrophils were infiltrated in human RCC lesions than those found in surrounding normal kidney tissues. Similarly, in vitro studies also revealed that RCC cells recruited more neutrophil HL-60N cells than normal kidney epithelial cells. Furthermore, in vitro and in vivo experiments also showed that the infiltrated neutrophils could promote RCC cell growth. Mechanism studies showed that co-culture of RCC cells with neutrophil HL-60N cells could selectively upregulate the androgen receptor (AR) signals, which might then alter the c-Myc signals. Interruption approaches using AR-siRNA to knock down AR in RCC cells blocked neutrophil-enhanced RCC cell proliferation. In vivo data using an orthotopically xenografted RCC mouse model also confirmed that infiltrated neutrophils could promote RCC proliferation via modulating the expressions of related cytokines. Together, these results conclude that infiltrated neutrophils may function through modulating the AR --> c-Myc signals to promote RCC cell proliferation. Targeting this newly identified infiltrating neutrophil --> AR --> c-Myc signal pathway in the kidney tumor microenvironment may provide a new potential therapy to better suppress RCC progression.
Discovery of inducible nitric oxide synthase (iNOS) inhibitor development candidate KD7332, part 1: Identification of a novel, potent, and selective series of quinolinone iNOS dimerization inhibitors that are orally active in rodent pain models.[Pubmed:19374401]
J Med Chem. 2009 May 14;52(9):3047-62.
There are three isoforms of dimeric nitric oxide synthases (NOS) that convert arginine to citrulline and nitric oxide. Inducible NOS is implicated in numerous inflammatory diseases and, more recently, in neuropathic pain states. The majority of existing NOS inhibitors are either based on the structure of arginine or are substrate competitive. We describe the identification from an ultra high-throughput screen of a novel series of quinolinone small molecule, nonarginine iNOS dimerization inhibitors. SAR studies on the screening hit, coupled with an in vivo lipopolysaccharide (LPS) challenge assay measuring plasma nitrates and drug levels, rapidly led to the identification of compounds 12 and 42--potent inhibitors of the human and mouse iNOS enzyme that were highly selective over endothelial NOS (eNOS). Following oral dosing, compounds 12 and 42 gave a statistical reduction in pain behaviors in the mouse formalin model, while 12 also statistically reduced neuropathic pain behaviors in the chronic constriction injury (Bennett) model.
Antinociceptive activity of the selective iNOS inhibitor AR-C102222 in rodent models of inflammatory, neuropathic and post-operative pain.[Pubmed:16125426]
Eur J Pain. 2006 Aug;10(6):505-12.
Nitric oxide generated by the nitric oxide synthase (NOS) isoforms contributes to pain processing. The selective inhibition of iNOS might represent a novel, therapeutic target for the development of antinociceptive compounds. However, few isoform-selective inhibitors of NOS have been developed. The present experiments examined the anti-inflammatory and antinociceptive activity of a selective inducible nitric oxide (iNOS) inhibitor, AR-C102222, on arachidonic acid-induced ear inflammation, Freund's complete adjuvant (FCA)-induced hyperalgesia, acetic acid-induced writhing, and tactile allodynia produced by L5 spinal nerve ligation (L5 SNL) or hindpaw incision (INC). AR-C102222 at a dose of 100mg/kg p.o., significantly reduced inflammation produced by the application of arachidonic acid to the ear, attenuated FCA-induced mechanical hyperalgesia, and attenuated acetic acid-induced writhing. In the L5 SNL and INC surgical procedures, tactile allodynia produced by both procedures was significantly reduced by 30mg/kg i.p. of AR-C102222. These data demonstrate that the selective inhibition of iNOS produces antinociception in different models of pain and suggest that the iNOS-NO system plays a role in pain processing.
1,2-Dihydro-4-quinazolinamines: potent, highly selective inhibitors of inducible nitric oxide synthase which show antiinflammatory activity in vivo.[Pubmed:12620067]
J Med Chem. 2003 Mar 13;46(6):913-6.
The discovery of a novel class of nitric oxide synthase (NOS) inhibitors, 2-substituted 1,2-dihydro-4-quinazolinamines, and the related 4'-aminospiro[piperidine-4,2'(1'H)-quinazolin]-4'-amines is described. Members of both series exhibit nanomolar potency and high selectivity for the inducible isoform of the enzyme (i-NOS) relative to the constitutive isoforms in vitro. Efficacy in acute and chronic animal models of inflammatory disease following oral administration has also been demonstrated using these compounds.


