AM 404Anandamide transport inhibitor CAS# 198022-70-7 |
- MLN2238
Catalog No.:BCC2092
CAS No.:1072833-77-2
- MG-115
Catalog No.:BCC1237
CAS No.:133407-86-0
- Salinosporamide A (NPI-0052, Marizomib)
Catalog No.:BCC2094
CAS No.:437742-34-2
- Gliotoxin
Catalog No.:BCN3894
CAS No.:67-99-2
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 198022-70-7 | SDF | Download SDF |
| PubChem ID | 6604822 | Appearance | Powder |
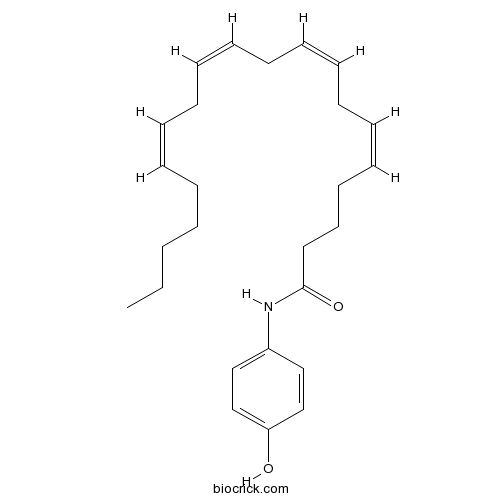
| Formula | C26H37NO2 | M.Wt | 395.59 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | AM-404;183718-77-6 | ||
| Solubility | Soluble to 50 mM in ethanol and to 50 mM in DMSO | ||
| Chemical Name | (5Z,8Z,11Z,14Z)-N-(4-hydroxyphenyl)icosa-5,8,11,14-tetraenamide | ||
| SMILES | CCCCCC=CCC=CCC=CCC=CCCCC(=O)NC1=CC=C(C=C1)O | ||
| Standard InChIKey | IJBZOOZRAXHERC-DOFZRALJSA-N | ||
| Standard InChI | InChI=1S/C26H37NO2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-26(29)27-24-20-22-25(28)23-21-24/h6-7,9-10,12-13,15-16,20-23,28H,2-5,8,11,14,17-19H2,1H3,(H,27,29)/b7-6-,10-9-,13-12-,16-15- | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Competitive and selective inhibitor of carrier-mediated anandamide transport (IC50 = 1 μM). Does not activate CB1 receptors or inhibit anandamide hydrolysis but has been shown to activate native and cloned vanilloid receptors (pEC50 = 7.4). Active in vivo. Also available in water soluble emulsion . |

AM 404 Dilution Calculator

AM 404 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5279 mL | 12.6393 mL | 25.2787 mL | 50.5574 mL | 63.1967 mL |
| 5 mM | 0.5056 mL | 2.5279 mL | 5.0557 mL | 10.1115 mL | 12.6393 mL |
| 10 mM | 0.2528 mL | 1.2639 mL | 2.5279 mL | 5.0557 mL | 6.3197 mL |
| 50 mM | 0.0506 mL | 0.2528 mL | 0.5056 mL | 1.0111 mL | 1.2639 mL |
| 100 mM | 0.0253 mL | 0.1264 mL | 0.2528 mL | 0.5056 mL | 0.632 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
AM 404 is a selective inhibitor of anandamide transport [1]. Also, it is an agonist of CB1 cannabinoid receptor and potential vanilloid type 1 (TRPV1).
The endocannabinoid transporter (eCBT) is a transporter for the endocannabinoid. The blockade of anandamide transport has anti-nociceptive effects.
AM 404 is a selective anandamide transport inhibitor. AM404 inhibited anandamide transport with IC50 values of 1 and 5 μM in neurons and astrocytes, respectively. However, AM404 had no effect on FAAH activity or on uptake of arachidonate or ethanolamine. Also, AM404 exhibited affinity for CB1 receptors with Ki value of 1.8 μM [1]. In rat hepatic artery contracted with phenylephrine, AM404 evoked relaxations in a concentration-dependent way, which was inhibited by capsazepine, a vanilloid receptor antagonist. These results suggested that AM404 activated vanilloid receptors [2]. In SK-N-SH neuroblastoma cells, AM404 inhibited NFAT and NF-κB signaling pathways [3].
In mice, AM404 (10 mg/kg) significantly prolonged and enhanced anandamide-induced analgesia [1]. In rats, AM404 inhibited motor behaviors induced by quinpirole, a D2 family receptor agonist. In juvenile spontaneously hypertensive rats, AM404 inhibited hyperactivity [4].
References:
[1]. Beltramo M, Stella N, Calignano A, et al. Functional role of high-affinity anandamide transport, as revealed by selective inhibition. Science, 1997, 277(5329): 1094-1097.
[2]. Zygmunt PM, Chuang H, Movahed P, et al. The anandamide transport inhibitor AM404 activates vanilloid receptors. Eur J Pharmacol, 2000, 396(1): 39-42.
[3]. Caballero FJ, Soler-Torronteras R, Lara-Chica M, et al. AM404 inhibits NFAT and NF-κB signaling pathways and impairs migration and invasiveness of neuroblastoma cells. Eur J Pharmacol, 2015, 746: 221-232.
[4]. Beltramo M, de Fonseca FR, Navarro M, et al. Reversal of dopamine D(2) receptor responses by an anandamide transport inhibitor. J Neurosci, 2000, 20(9): 3401-3407.
- GW311616 hydrochloride
Catalog No.:BCC5394
CAS No.:197890-44-1
- Stachartin E
Catalog No.:BCN6970
CAS No.:1978388-58-7
- Stachartin D
Catalog No.:BCN6971
CAS No.:1978388-57-6
- Stachartin C
Catalog No.:BCN6972
CAS No.:1978388-56-5
- Stachartin B
Catalog No.:BCN6973
CAS No.:1978388-55-4
- Stachartin A
Catalog No.:BCN6974
CAS No.:1978388-54-3
- SN 003
Catalog No.:BCC7633
CAS No.:197801-88-0
- TFLLR-NH2
Catalog No.:BCC3948
CAS No.:197794-83-5
- 7-O-Acetylbonducellpin C
Catalog No.:BCN7558
CAS No.:197781-86-5
- Bonducellpin D
Catalog No.:BCN7544
CAS No.:197781-85-4
- Bonducellpin C
Catalog No.:BCN7647
CAS No.:197781-84-3
- Daphmacrine
Catalog No.:BCN4868
CAS No.:19775-48-5
- GW311616
Catalog No.:BCC5393
CAS No.:198062-54-3
- Triptobenzene K
Catalog No.:BCN8055
CAS No.:198129-88-3
- Gap 27
Catalog No.:BCC1033
CAS No.:198284-64-9
- Medicagol
Catalog No.:BCN8430
CAS No.:1983-72-8
- Myricetin 3-O-beta-D-glucopyranoside
Catalog No.:BCN8144
CAS No.:19833-12-6
- Erythrodiol 3-palmitate
Catalog No.:BCN4869
CAS No.:19833-13-7
- LY 367385
Catalog No.:BCC6983
CAS No.:198419-91-9
- Boc-D-Pen(pMeBzl)-OH.DCHA
Catalog No.:BCC3308
CAS No.:198470-36-9
- Parecoxib
Catalog No.:BCC4041
CAS No.:198470-84-7
- Parecoxib Sodium
Catalog No.:BCC4248
CAS No.:198470-85-8
- Boc-Pen(pMeBzl)-OH.DCHA
Catalog No.:BCC2623
CAS No.:198474-61-2
- Bazedoxifene HCl
Catalog No.:BCC4492
CAS No.:198480-56-7
Delta9-tetrahydrocannabinol (THC) and AM 404 protect against cerebral ischaemia in gerbils through a mechanism involving cannabinoid and opioid receptors.[Pubmed:17965746]
Br J Pharmacol. 2007 Dec;152(8):1301-11.
BACKGROUND AND PURPOSE: It has been suggested that the endocannabinoid system elicits neuroprotection against excitotoxic brain damage. In the present study the therapeutic potential of AM 404 on ischaemia-induced neuronal injury was investigated in vivo and compared with that of the classical cannabinoid receptor type 1 (CB1) agonist, delta 9-tetraydrocannabinol (THC), using a model of transient global cerebral ischaemia in the gerbil. EXPERIMENTAL APPROACH: The effects of AM 404 (0.015-2 mg kg(-1)) and THC (0.05-2 mg kg(-1)), given 5 min after ischaemia, were measured from 1 h to 7 days in terms of electroencephalographic (EEG) total spectral power, spontaneous motor activity, memory function, rectal temperature and hippocampal CA1 neuronal count. KEY RESULTS: Over the dose range tested, AM 404 (2 mg kg(-1)) and THC (1 mg kg(-1)) completely reversed the ischaemia-induced behavioural, EEG and histological damage. Only THC (1 and 2 mg kg(-1)) induced a decrease of body temperature. Pretreatment with the selective CB1 receptor antagonist, AM 251 (1 mg kg(-1)) and the opioid antagonist, naloxone (2 mg kg(-1)) reversed the protective effect induced by both AM 404 and THC while the TRPV1 vanilloid antagonist, capsazepine (0.01 mg kg(-1)), was ineffective. CONCLUSIONS AND IMPLICATIONS: Our findings demonstrate that AM 404 and THC reduce neuronal damage caused by bilateral carotid occlusion in gerbils and that this protection is mediated through an interaction with CB1 and opioid receptors. Endocannabinoids might form the basis for the development of new neuroprotective drugs useful for the treatment of stroke and other neurodegenerative pathologies.
AM-404 elevates renal intracellular Ca(2+), questioning its selectivity as a pharmacological tool for investigating the anandamide transporter.[Pubmed:11755382]
J Pharmacol Toxicol Methods. 2001 May-Jun;45(3):195-8.
The effect of N-(4-hydroxyphenyl)-arachidonamide (AM-404), a drug commonly used to inhibit the anandamide transporter, on intracellular free Ca(2+) levels ([Ca(2+)](i)) was studied in Madin Darby canine kidney (MDCK) cells. [Ca(2+)](i) was measured using fura-2 as a Ca(2+) indicator. Between 2 and 40 microM, AM-404 increased [Ca(2+)](i) in a concentration-dependent fashion with an EC(50) value of 20 microM. Removal of extracellular Ca(2+) abolished the [Ca(2+)](i) signals. The [Ca(2+)](i) increase was nearly abrogated by 10 microM La(3+), but was insensitive to 50 microM Ni(2+) and 10 microM of nifedipine, nimodipine, nicardipine, and verapamil. At a concentration that did not increase [Ca(2+)](i), AM-404 (1 microM) did not alter the [Ca(2+)](i) increases induced by 10 microM ATP and 1 microM bradykinin. AM-404 (5 microM) also increased [Ca(2+)](i) in Chang liver cells, PC3 human prostate cancer cells, BFTC human bladder cancer cells, and MG63 human osteoblast-like cells. Together, this study shows for the first time that AM-404 at concentrations commonly used to inhibit the anandamide transporter in various systems induced an increase in [Ca(2+)](i) in different cell types. The [Ca(2+)](i) increase was solely due to extracellular Ca(2+) influx. Thus caution must be exercised in using AM-404 as a selective inhibitor of the anandamide transporter.
Role of TRPV1 and cannabinoid CB1 receptors in AM 404-evoked hypothermia in rats.[Pubmed:16647109]
Pharmacol Biochem Behav. 2006 Apr;83(4):508-16.
AM 404 inhibits endocannabinoid uptake and enhances the cannabinoid CB(1)-mediated effects of endogenous cannabinoids. Accumulating evidence also suggests that AM 404 acts at sites other than the endocannabinoid system. One site is the transient receptor potential vanilloid 1 cation channel (TRPV1). A useful endpoint for discriminating between TRPV1- or CB(1)-mediated effects of AM 404 is hypothermia. This is because TRPV1 or CB(1) receptor activation produces a significant hypothermia in rats. The present study investigated the effects of AM 404 (1, 5, 10 and 20 mg/kg, i.p.) on body temperature in rats and the involvement of TRPV1 and CB(1) receptors in the effects of AM 404. Doses of 10 and 20 mg/kg of AM 404 produced significant hypothermia. Pre-treatment with capsazepine (30 mg/kg, i.p.) blocked the hypothermia caused by 10 and 20 mg/kg of AM 404. Pre-treatment with SB 366791 (2 mg/kg, i.p.), a new TRPV1 antagonist, also abolished the hypothermia evoked by AM 404 (20 mg/kg, i.p.). In contrast, pre-treatment with SR 141716A (Rimonabant), a CB(1) antagonist, or AA-5-HT, a fatty acid amide hydrolase (FAAH) blocker, did not affect AM 404-evoked hypothermia. The present data demonstrate that AM 404 evokes a significant hypothermia in rats that is dependent on TRPV1 receptor activation.
5-HT1A receptors are involved in the anxiolytic effect of Delta9-tetrahydrocannabinol and AM 404, the anandamide transport inhibitor, in Sprague-Dawley rats.[Pubmed:17116299]
Eur J Pharmacol. 2007 Jan 26;555(2-3):156-63.
The mechanism mediating the effects of cannabinoids on anxiety-related responses appear to involve cannabinoid CB1 and non-CB1 receptors. However, other neurotransmitters may play a role in such effect. This study shows evidence of an interaction between endocannabinoid system and serotonin (5-HT), 1A receptor subtype on anxiety-like behavior in Sprague-Dawley rats. The exogenous cannabinoid agonist, Delta9-tetrahydrocannabinol (THC), and N-(4-hydroxyphenyl)-arachidonylamide, the anandamide transporter inhibitor (AM 404) were evaluated in the elevated plus maze test. THC (0.075-0.75 mg/kg i.p.), given 30 min and AM 404 (0.75-1.25 mg/kg i. p.), given 60 min before the test, exhibited a dose-response anxiolytic effect evaluated in terms of increase in the percentage of total entries and time spent in the open and decrease of total entries and time spent in the closed arms. The anxiolytic effect obtained with the maximal active dose of both THC (0.75 mg/kg) and AM 404 (1.25 mg/kg) was blocked by the 5-HT1A receptor antagonist, N-[2-[4-(2-methoxyphenyl) piperazin-1-yl]ethyl]-N-pyridin-2-yl-cyclohexanecarboxamide dihydro chloride (WAY-100635 (300 microg/kg, s.c.), given 30 min before THC or 15 min before AM 404. The combination of an ineffective dose of THC (0.015 mg/kg) or AM 404 (0.015 mg/kg) on anxiety-related responses with an ineffective dose of the 5HT(1A) receptor agonist, 8-Hydroxy-2-(di-n-propylamino) tetralin hydrobromide (8-OH-DPAT) (7.5 microg/kg, i.p.), led to a synergistic effect. No interference with spontaneous motor activity, evaluated in an activity cage for 5 min, in rats given the drugs alone or in combination, was found. These data suggest that the anxiolytic effect produced by endo- and eso-cannabinoids is modulated by 5-HT1A receptors.
The anandamide transport inhibitor AM404 activates vanilloid receptors.[Pubmed:10822052]
Eur J Pharmacol. 2000 May 12;396(1):39-42.
The possibility that the anandamide transport inhibitor N-(4-hydroxyphenyl)-5,8,11,14-eicosatetraenamide (AM404), structurally similar to the vanilloid receptor agonists anandamide and capsaicin, may also activate vanilloid receptors and cause vasodilation was examined. AM404 evoked concentration-dependent relaxations in segments of rat isolated hepatic artery contracted with phenylephrine. Relaxations were abolished in preparations pre-treated with capsaicin. The calcitonin-gene related peptide (CGRP) receptor antagonist CGRP-(8-37) also abolished relaxations. The vanilloid receptor antagonist capsazepine inhibited vasodilation by AM404 and blocked AM404-induced currents in patch-clamp experiments on Xenopus oocytes expressing the vanilloid subtype 1 receptor (VR1). In conclusion, AM404 activates native and cloned vanilloid receptors.
Structural determinants for recognition and translocation by the anandamide transporter.[Pubmed:10318965]
Proc Natl Acad Sci U S A. 1999 May 11;96(10):5802-7.
The biological actions of anandamide (arachidonylethanolamide), an endogenous cannabinoid lipid, are terminated by a two-step inactivation process consisting of carrier-mediated uptake and intracellular hydrolysis. Anandamide uptake in neurons and astrocytes is mediated by a high-affinity, Na+-independent transporter that is selectively inhibited by N-(4-hydroxyphenyl)-arachidonamide (AM404). In the present study, we examined the structural determinants governing recognition and translocation of substrates by the anandamide transporter constitutively expressed in a human astrocytoma cell line. Competition experiments with a select group of analogs suggest that substrate recognition by the transporter is favored by a polar nonionizable head group of defined stereochemical configuration containing a hydroxyl moiety at its distal end. The secondary carboxamide group interacts favorably with the transporter, but may be replaced with either a tertiary amide or an ester, suggesting that it may serve as hydrogen acceptor. Thus, 2-arachidonylglycerol, a putative endogenous cannabinoid ester, also may serve as a substrate for the transporter. Substrate recognition requires the presence of at least one cis double bond situated at the middle of the fatty acid carbon chain, indicating a preference for ligands whose hydrophobic tail can adopt a bent U-shaped conformation. On the other hand, uptake experiments with radioactively labeled substrates show that no fewer than four cis nonconjugated double bonds are required for optimal translocation across the cell membrane, suggesting that substrates are transported in a folded hairpin conformation. These results outline the general structural requisites for anandamide transport and may assist in the development of selective inhibitors with potential clinical applications.
Functional role of high-affinity anandamide transport, as revealed by selective inhibition.[Pubmed:9262477]
Science. 1997 Aug 22;277(5329):1094-7.
Anandamide, an endogenous ligand for central cannabinoid receptors, is released from neurons on depolarization and rapidly inactivated. Anandamide inactivation is not completely understood, but it may occur by transport into cells or by enzymatic hydrolysis. The compound N-(4-hydroxyphenyl)arachidonylamide (AM404) was shown to inhibit high-affinity anandamide accumulation in rat neurons and astrocytes in vitro, an indication that this accumulation resulted from carrier-mediated transport. Although AM404 did not activate cannabinoid receptors or inhibit anandamide hydrolysis, it enhanced receptor-mediated anandamide responses in vitro and in vivo. The data indicate that carrier-mediated transport may be essential for termination of the biological effects of anandamide, and may represent a potential drug target.
Potentiation of anandamide hypotension by the transport inhibitor, AM404.[Pubmed:9389389]
Eur J Pharmacol. 1997 Oct 15;337(1):R1-2.
The putative endogenous cannabinoid, anandamide (0.2-2 mg/kg i.v.), decreased systemic blood pressure dose-dependently in anesthesized guinea pigs. These effects were prevented by the CB1 cannabinoid receptor antagonist SR141716A [N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-me thyl-1H-pyrazole-3-carboxamide x HCl] at the dose of 0.2 mg/kg i.v. The vasodepressor responses to anandamide were significantly potentiated and prolonged by a novel inhibitor of carrier-mediated anandamide transport, N-(4-hydroxyphenyl) arachidonylethanolamide (AM404) (10 mg/kg, i.v.). These results suggest that anandamide transport participates in terminating the vascular actions of anandamide.


