A 841720mGluR1 antagonist, non-competitive CAS# 869802-58-4 |
- SB 431542
Catalog No.:BCC3658
CAS No.:301836-41-9
- SB-505124 hydrochloride
Catalog No.:BCC1930
CAS No.:356559-13-2
- SB525334
Catalog No.:BCC2531
CAS No.:356559-20-1
- SD-208
Catalog No.:BCC1938
CAS No.:627536-09-8
- LY2109761
Catalog No.:BCC3806
CAS No.:700874-71-1
- LY2157299
Catalog No.:BCC3709
CAS No.:700874-72-2
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 869802-58-4 | SDF | Download SDF |
| PubChem ID | 11559235 | Appearance | Powder |
| Formula | C17H21N5OS | M.Wt | 343.45 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 50 mM in DMSO and to 50 mM in ethanol | ||
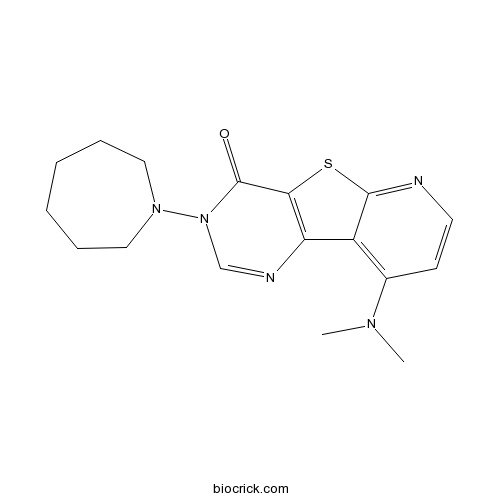
| Chemical Name | 3-(azepan-1-yl)-9-(dimethylamino)pyrido[1,2]thieno[3,4-d]pyrimidin-4-one | ||
| SMILES | CN(C)C1=C2C3=C(C(=O)N(C=N3)N4CCCCCC4)SC2=NC=C1 | ||
| Standard InChIKey | GYWGXEGOXODOQU-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C17H21N5OS/c1-20(2)12-7-8-18-16-13(12)14-15(24-16)17(23)22(11-19-14)21-9-5-3-4-6-10-21/h7-8,11H,3-6,9-10H2,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent, non-competitive mGlu1 receptor antagonist that displays 34-fold selectivity over mGlu5 (IC50 values are 10 and 342 nM respectively). Displays no significant activity at a range of other GPCRs, ion channels and transporters. Exhibits analgesic effects; decreases mechanical allodynia in models of neuropathic pain. Also impairs cognitive function. |

A 841720 Dilution Calculator

A 841720 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.9116 mL | 14.5582 mL | 29.1163 mL | 58.2326 mL | 72.7908 mL |
| 5 mM | 0.5823 mL | 2.9116 mL | 5.8233 mL | 11.6465 mL | 14.5582 mL |
| 10 mM | 0.2912 mL | 1.4558 mL | 2.9116 mL | 5.8233 mL | 7.2791 mL |
| 50 mM | 0.0582 mL | 0.2912 mL | 0.5823 mL | 1.1647 mL | 1.4558 mL |
| 100 mM | 0.0291 mL | 0.1456 mL | 0.2912 mL | 0.5823 mL | 0.7279 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
A 841720 is a novel non-competitive antagonist of metabotropic glutamate receptor 1 (mGluR1), with an IC50 value of 10.7 nM to 10 µM L-glutamate-induced calcium release at human mGluR1 receptors [1].
Glutamate as the major excitatory neurotransmitter in the central nervous system functions through two types of receptors, ionotropic glutamate receptors and metabotropic glutamate receptors (mGluRs). MGluRs includes group I mGluRs (mGluR1 and mGluR5 receptors), group II (mGluR2 and mGluR3 receptors) and group III (mGluR4, 6, 7, 8 receptors) [1].
In cells, agonist-induced calcium release was concentration-dependently inhibited by A 841720 in a human mGluR5 receptor FLIPR functional assay. But the IC50 value was just 343 nM. In cells expressing recombinant mGluR5 receptors, A 841720 did not block agonist-induced response. In the human mGluR1 receptor FLIPR assay, the log concentration-response curve was shifted by A 841720 at 10 nM to the right. A 841720 at increasing concentrations profoundly reduced the amplitude of L-quisqualate-evoked calcium release. A 841720 at 30 nM reduced the maximal agonist-induced response by 38%. L-quisqualate-induced response was completely abolished by A 841720 at 100 nM [1].
In a water maze test, all rats gradually learned to locate the submerged platform. Treatment with A 841720 significantly slowered rats to find the platform than vehicle control rats. Rats treated with A 841720 at both 30 and 100 µmol/kg doses not only significantly traveled longer distance to find the hidden platform, but also significantly spent longer time to reach the platform [1].
Reference:
[1]. El-Kouhen O, Lehto SG, Pan JB, et al. Blockade of mGluR1 receptor results in analgesia and disruption of motor and cognitive performances: effects of A-841720, a novel non-competitive mGluR1 receptor antagonist. British journal of pharmacology, 2006, 149(6): 761-774.
- threo-Guaiacylglycerol beta-coniferyl ether
Catalog No.:BCN1323
CAS No.:869799-76-8
- A-770041
Catalog No.:BCC1323
CAS No.:869748-10-7
- Obestatin (rat)
Catalog No.:BCC5912
CAS No.:869705-22-6
- Fmoc-Tyr(tBu)-ol
Catalog No.:BCC2572
CAS No.:86967-51-3
- Tecovirimat
Catalog No.:BCC5518
CAS No.:869572-92-9
- JNJ 10191584 maleate
Catalog No.:BCC7362
CAS No.:869497-75-6
- Praeroside II
Catalog No.:BCN7001
CAS No.:86940-46-7
- 14-Deoxy-17-hydroxyandrographolide
Catalog No.:BCN4560
CAS No.:869384-82-7
- MLN8054
Catalog No.:BCC2170
CAS No.:869363-13-3
- AZD8330
Catalog No.:BCC3733
CAS No.:869357-68-6
- Neurokinin B (human, porcine)
Catalog No.:BCC7119
CAS No.:86933-75-7
- Neurokinin A (porcine)
Catalog No.:BCC6955
CAS No.:86933-74-6
- Andropanolide
Catalog No.:BCN4559
CAS No.:869807-57-8
- Formoxanthone A
Catalog No.:BCN6451
CAS No.:869880-32-0
- Radezolid
Catalog No.:BCC1882
CAS No.:869884-78-6
- VRT752271
Catalog No.:BCC4122
CAS No.:869886-67-9
- Alpinumisoflavone acetate
Catalog No.:BCN6813
CAS No.:86989-18-6
- MK-2048
Catalog No.:BCC2136
CAS No.:869901-69-9
- TLQP 21
Catalog No.:BCC2405
CAS No.:869988-94-3
- Thiolutin
Catalog No.:BCC2471
CAS No.:87-11-6
- Salicylanilide
Catalog No.:BCC4712
CAS No.:87-17-2
- Ac-DL-Trp-OH
Catalog No.:BCC3119
CAS No.:87-32-1
- Isosorbide dinitrate
Catalog No.:BCC9004
CAS No.:87-33-2
- trans-Caryophyllene
Catalog No.:BCN2644
CAS No.:87-44-5
Comparison of the mGluR1 antagonist A-841720 in rat models of pain and cognition.[Pubmed:17551319]
Behav Pharmacol. 2007 Jul;18(4):273-81.
In the current study we compared the potency of the selective metabotropic glutamate receptor (mGluR1) antagonist A-841720 (7-Azepan-1-yl-4-dimethylamino-7H-9-thia-1,5,7-triaza-fluoren-8-one) in rodent models of pain with its effects in models of learning and memory, to obtain information regarding the therapeutic window of this compound. A-841720 significantly reduced formalin-induced behaviours and complete Freund's adjuvant (CFA)-induced tactile allodynia, starting at doses of 1 and 10 mg/kg, respectively. At the dose of 3 mg/kg, however, A-841720 significantly reduced the percentage of spontaneous alternations in a radial-maze task. In contextual-fear conditioning, A-841720, given at the dose of 10 mg/kg before acquisition, significantly reduced freezing behaviour tested 24 h later. In the same task, repeated treatment for 5 days did not reduce the impairing effect of the challenge dose, indicating a lack of tolerance development. In a passive-avoidance task, A-841720 at 10 mg/kg administered before acquisition, significantly reduced the latency to enter the dark box on the retention test. Given that complete Freund's adjuvant is a better measure of analgesia, these results indicate that the selective mGluR1 antagonist A-841720 has analgesic potential in a dose range at which it also produces memory impairments. This diminishes its therapeutic potential for the treatment of chronic pain.
Blockade of mGluR1 receptor results in analgesia and disruption of motor and cognitive performances: effects of A-841720, a novel non-competitive mGluR1 receptor antagonist.[Pubmed:17016515]
Br J Pharmacol. 2006 Nov;149(6):761-74.
BACKGROUND AND PURPOSE: To further assess the clinical potential of the blockade of metabotropic glutamate receptors (mGluR1) for the treatment of pain. EXPERIMENTAL APPROACH: We characterized the effects of A-841720, a novel, potent and non-competitive mGluR1 antagonist in models of pain and of motor and cognitive function. KEY RESULTS: At recombinant human and native rat mGluR1 receptors, A-841720 inhibited agonist-induced calcium mobilization, with IC50 values of 10.7+/-3.9 and 1.0 +/- 0.2 nM, respectively, while showing selectivity over other mGluR receptors, in addition to other neurotransmitter receptors, ion channels, and transporters. Intraperitoneal injection of A-841720 potently reduced complete Freund's adjuvant-induced inflammatory pain (ED50 = 23 micromol kg(-1)) and monoiodoacetate-induced joint pain (ED50 = 43 micromol kg(-1)). A-841720 also decreased mechanical allodynia observed in both the sciatic nerve chronic constriction injury and L5-L6 spinal nerve ligation (SNL) models of neuropathic pain (ED50 = 28 and 27 micromol kg(-1), respectively). Electrophysiological studies demonstrated that systemic administration of A-841720 in SNL animals significantly reduced evoked firing in spinal wide dynamic range neurons. Significant motor side effects were observed at analgesic doses and A-841720 also impaired cognitive function in the Y-maze and the Water Maze tests. CONCLUSIONS AND IMPLICATIONS: The analgesic effects of a selective mGluR1 receptor antagonist are associated with motor and cognitive side effects. The lack of separation between efficacy and side effects in pre-clinical models indicates that mGluR1 antagonism may not provide an adequate therapeutic window for the development of such antagonists as novel analgesic agents in humans.
Structure-activity relationship of triazafluorenone derivatives as potent and selective mGluR1 antagonists.[Pubmed:16279797]
J Med Chem. 2005 Nov 17;48(23):7374-88.
SAR (structure-activity relationship) studies of triazafluorenone derivatives as potent mGluR1 antagonists are described. The triazafluorenone derivatives are non-amino acid derivatives and noncompetitive mGluR1 antagonists that bind at a putative allosteric recognition site located within the seven-transmembrane domain of the receptor. These triazafluorenone derivatives are potent, selective, and systemically active mGluR1 antagonists. Compound 1n, for example, was a very potent mGluR1 antagonist (IC50 = 3 nM) and demonstrated full efficacy in various in vivo animal pain models.


