7,8,9,9-TetradehydroisolariciresinolCAS# 145918-59-8 |
Quality Control & MSDS
Number of papers citing our products

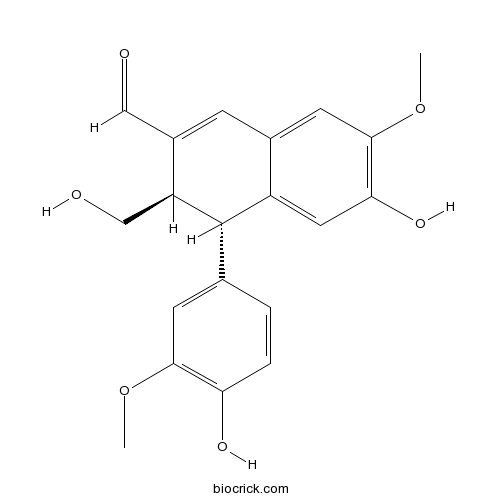
Chemical structure

3D structure
| Cas No. | 145918-59-8 | SDF | Download SDF |
| PubChem ID | 9998370 | Appearance | Powder |
| Formula | C20H20O6 | M.Wt | 356.4 |
| Type of Compound | Lignans | Storage | Desiccate at -20°C |
| Synonyms | 357645-16-0 | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (3R,4S)-6-hydroxy-4-(4-hydroxy-3-methoxyphenyl)-3-(hydroxymethyl)-7-methoxy-3,4-dihydronaphthalene-2-carbaldehyde | ||
| SMILES | COC1=C(C=CC(=C1)C2C(C(=CC3=CC(=C(C=C23)O)OC)C=O)CO)O | ||
| Standard InChIKey | PATMJUOZIPKVAS-YWZLYKJASA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Targets | NO |

7,8,9,9-Tetradehydroisolariciresinol Dilution Calculator

7,8,9,9-Tetradehydroisolariciresinol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8058 mL | 14.0292 mL | 28.0584 mL | 56.1167 mL | 70.1459 mL |
| 5 mM | 0.5612 mL | 2.8058 mL | 5.6117 mL | 11.2233 mL | 14.0292 mL |
| 10 mM | 0.2806 mL | 1.4029 mL | 2.8058 mL | 5.6117 mL | 7.0146 mL |
| 50 mM | 0.0561 mL | 0.2806 mL | 0.5612 mL | 1.1223 mL | 1.4029 mL |
| 100 mM | 0.0281 mL | 0.1403 mL | 0.2806 mL | 0.5612 mL | 0.7015 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- CGP 53353
Catalog No.:BCC7363
CAS No.:145915-60-2
- CGP 52411
Catalog No.:BCC7667
CAS No.:145915-58-8
- Brachynoside
Catalog No.:BCN3749
CAS No.:145898-87-9
- D-myo-Inositol-1,3,4,5-tetrakisphosphate, octapotassium salt
Catalog No.:BCC7058
CAS No.:145843-69-2
- Tiagabine hydrochloride
Catalog No.:BCC5217
CAS No.:145821-59-6
- Margatoxin
Catalog No.:BCC7709
CAS No.:145808-47-5
- G-749
Catalog No.:BCC4009
CAS No.:1457983-28-6
- 4,6-Dichloro-2-(propylthio)pyrimidin-5-amine
Catalog No.:BCC8666
CAS No.:145783-15-9
- 4,6-Dichloro-5-nitro-2-propylthiopyrimidine
Catalog No.:BCC8667
CAS No.:145783-14-8
- AR-C 66096 tetrasodium salt
Catalog No.:BCC6004
CAS No.:145782-74-7
- 3-Allylrhodanine
Catalog No.:BCC8604
CAS No.:1457-47-2
- HG-9-91-01
Catalog No.:BCC4071
CAS No.:1456858-58-4
- 3-Amino-3-phenyl-1-propanol
Catalog No.:BCC8608
CAS No.:14593-04-5
- 2-(Methylamino)ethylphosphonic acid
Catalog No.:BCN1763
CAS No.:14596-55-5
- 2-Dimethylaminoethylphosphonic acid
Catalog No.:BCN1764
CAS No.:14596-56-6
- N,N,N-Trimethyl-2-aminoethylphosphonate
Catalog No.:BCN1560
CAS No.:14596-57-7
- Laccaic acid E
Catalog No.:BCN1807
CAS No.:14597-16-1
- Jasminoid A
Catalog No.:BCN7605
CAS No.:1459784-57-6
- Cycloart-23-ene-3,25-diol
Catalog No.:BCN2640
CAS No.:14599-48-5
- Yohimbine
Catalog No.:BCN2293
CAS No.:146-48-5
- 2-Chloroadenosine
Catalog No.:BCC7575
CAS No.:146-77-0
- 2-Fluoroadenosine
Catalog No.:BCC8576
CAS No.:146-78-1
- Tropine nonanoate
Catalog No.:BCN1925
CAS No.:146018-90-8
- SC 51322
Catalog No.:BCC5941
CAS No.:146032-79-3
Higher Expression of Toll-like Receptors 3, 7, 8, and 9 in Pityriasis Rosea.[Pubmed:28192646]
J Pathol Transl Med. 2017 Mar;51(2):148-151.
BACKGROUND: Pityriasis rosea (PR) is a common papulosquamous skin disease in which an infective agent may be implicated. Toll-like receptors (TLRs) play an important role in immune responses and in the pathophysiology of inflammatory skin diseases. Our aim was to determine the possible roles of TLRs 3, 7, 8, and 9 in the pathogenesis of PR. METHODS: Twenty-four PR patients and 24 healthy individuals (as controls) were included in this case control study. All recruits were subjected to routine laboratory investigations. Biopsies were obtained from one active PR lesion and from healthy skin of controls for the detection of TLR 3, 7, 8, and 9 gene expression using real-time polymerase chain reaction. RESULTS: This study included 24 patients (8 females and 16 males) with active PR lesions, with a mean age of 28.62 years. Twenty four healthy age- and sex-matched individuals were included as controls (8 females and 16 males, with a mean age of 30.83 years). The results of the routine laboratory tests revealed no significant differences between both groups. Significantly elevated expression of all studied TLRs were detected in PR patients relative to healthy controls (p < .001). CONCLUSIONS: TLRs 3, 7, 8, and 9 might be involved in the pathogenesis of PR.
A Unified Approach to the Isomeric alpha-, beta-, gamma-, and delta-Carbolines via their 6,7,8,9-Tetrahydro Counterparts.[Pubmed:28304164]
J Org Chem. 2017 Apr 21;82(8):4328-4335.
A cross-coupling/reductive cyclization protocol has been employed in a unified approach to all four carbolines. So, for example, the 2-nitropyridine 8, which is readily prepared through an efficient palladium-catalyzed Ullmann cross-coupling reaction, is reductively cyclized under conventional conditions to give 6,7,8,9-tetrahydro-alpha-carboline that is itself readily aromatized to give alpha-carboline (1).
Pharmacological evaluation of 9,10-dihydrochromeno[8,7-e][1,3]oxazin-2(8H)-one derivatives as potent anti-inflammatory agent.[Pubmed:28273501]
Pharmacol Rep. 2017 Jun;69(3):419-425.
BACKGROUND: Non-steroidal anti-inflammatory drugs (NSAIDs) are the most widely administered drugs for the treatment of inflammation. However, they usually cause some unexpected side effects. Coumarins and their derivatives exhibit broad-spectrum biological activities. In order to develop new anti-inflammatory drugs with high anti-inflammatory activity and less side effects, a series of 9-substituted-9,10-dihydrochromeno[8,7-e][1,3]oxazin-2(8H)-one derivatives were designed, synthesized, and screened for their anti-inflammatory activities. METHODS: We investigated the effect of compound 9-(2-chlorophenyl)-9,10-dihydrochromeno[8,7-e][1,3]oxazin-2(8H)-one (B3) on lipopolysaccharide (LPS)-induced cytokine levels in RAW 264.7 cells at concentrations between 6.25mug/ml and 25mug/ml. Concentrations of tumor necrosis factor-alpha (TNF-alpha) and interleukin-6 (IL-6) were measured by enzyme-linked immunosorbent assay (ELISA). Moreover, mitogen-activated protein kinase (MAPK) and nuclear factor-kappaB (NF-kappaB) activation was investigated by western blot assay. RESULTS: Compound B3 could inhibit inflammatory responses via suppression of the NF-kappaB and MAPK signaling pathways. Docking study of the prepared compounds was performed for the study of interaction of molecules with the active site of TNF-alpha. CONCLUSION: 9,10-Dihydrochromeno[8,7-e][1,3]oxazin-2(8H)-one derivatives showed anti-inflammatory activity. Compound B3 was the most potent. The results of this study are encouraging further investigations to develop compound B3 as a novel therapeutic agent for inflammatory disorders.
Crystal structure of 9,20-dimethyl-1,8,12,19-tetra-aza-tetra-cyclo-[17.3.1.0(2,7).0(13,18)]tricosane dihydrate from synchrotron X-ray data.[Pubmed:28316816]
Acta Crystallogr E Crystallogr Commun. 2017 Feb 17;73(Pt 3):387-389.
The structure of the title compound, C21H40N4.2H2O, has been determined from synchrotron X-ray radiation data. The asymmetric unit comprises one 12-membered macropolycycle and two lattice water mol-ecules. The macropolycycle contains two cyclo-hexane rings and one 1,3-di-aza-cyclo-hexane ring, all in chair conformations. The C-N and C-C bond lengths are in the ranges 1.4526 (16)-1.4786 (17) and 1.517 (2)-1.5414 (17) A, respectively. One intra-molecular N-Hcdots, three dots, centeredN hydrogen bond helps to stabilize the mol-ecular conformation while medium-strength inter-molecular N-Hcdots, three dots, centeredO, O-Hcdots, three dots, centeredN and O-Hcdots, three dots, centeredO hydrogen bonds involving the lattice water mol-ecules connect the components into a three-dimensional network.


