5-Iodo-A-85380 dihydrochlorideCAS# 1217837-17-6 |
- Zebularine
Catalog No.:BCC1136
CAS No.:3690-10-6
- RG 108
Catalog No.:BCC1134
CAS No.:48208-26-0
- Nanaomycin A
Catalog No.:BCC3611
CAS No.:52934-83-5
- SGI-110
Catalog No.:BCC2221
CAS No.:929901-49-5
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 1217837-17-6 | SDF | Download SDF |
| PubChem ID | 54758463 | Appearance | Powder |
| Formula | C9H13Cl2IN2O | M.Wt | 363.03 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in water and to 100 mM in DMSO | ||
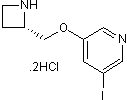
| Chemical Name | 3-[[(2S)-azetidin-2-yl]methoxy]-5-iodopyridine;dihydrochloride | ||
| SMILES | C1CNC1COC2=CC(=CN=C2)I.Cl.Cl | ||
| Standard InChIKey | ZJVVFYWEBSHGBV-JZGIKJSDSA-N | ||
| Standard InChI | InChI=1S/C9H11IN2O.2ClH/c10-7-3-9(5-11-4-7)13-6-8-1-2-12-8;;/h3-5,8,12H,1-2,6H2;2*1H/t8-;;/m0../s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | A highly potent and subtype-selective agonist for the α4β2 and α6β2 nicotinic acetylcholine receptors. Activates α-CTx-MII-sensitive and -insensitive components of [3H]dopamine release from rat striatal synaptosomes, corresponding to α6β2 and α4β2 (EC50 values are 12.7 and ~35 nM respectively). ~5000- , 25000- and 140000-fold selective over α3β4, α7 and muscle nAChR receptors respectively. Precursor also available. |

5-Iodo-A-85380 dihydrochloride Dilution Calculator

5-Iodo-A-85380 dihydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7546 mL | 13.773 mL | 27.5459 mL | 55.0919 mL | 68.8648 mL |
| 5 mM | 0.5509 mL | 2.7546 mL | 5.5092 mL | 11.0184 mL | 13.773 mL |
| 10 mM | 0.2755 mL | 1.3773 mL | 2.7546 mL | 5.5092 mL | 6.8865 mL |
| 50 mM | 0.0551 mL | 0.2755 mL | 0.5509 mL | 1.1018 mL | 1.3773 mL |
| 100 mM | 0.0275 mL | 0.1377 mL | 0.2755 mL | 0.5509 mL | 0.6886 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- RS 16566 dihydrochloride
Catalog No.:BCC6890
CAS No.:1217788-97-0
- SB 258719 hydrochloride
Catalog No.:BCC5937
CAS No.:1217674-10-6
- SB 205607 dihydrobromide
Catalog No.:BCC5687
CAS No.:1217628-73-3
- BYL-719
Catalog No.:BCC3707
CAS No.:1217486-61-7
- threo-1-C-Syringylglycerol
Catalog No.:BCN6110
CAS No.:121748-11-6
- NAS-181
Catalog No.:BCC7056
CAS No.:1217474-40-2
- (+)-UH 232 maleate
Catalog No.:BCC6790
CAS No.:1217473-50-1
- Isochandalone
Catalog No.:BCN4767
CAS No.:121747-90-8
- Isoderrone
Catalog No.:BCN3698
CAS No.:121747-89-5
- Ro 26-4550 trifluoroacetate
Catalog No.:BCC5813
CAS No.:1217448-66-2
- VD2-D3
Catalog No.:BCC2034
CAS No.:1217448-46-8
- A 350619 hydrochloride
Catalog No.:BCC5939
CAS No.:1217201-17-6
- Ac-Trp-OH
Catalog No.:BCC3109
CAS No.:1218-34-4
- Xylometazoline HCl
Catalog No.:BCC4879
CAS No.:1218-35-5
- Pidotimod
Catalog No.:BCC4823
CAS No.:121808-62-6
- CAY10505
Catalog No.:BCC4990
CAS No.:1218777-13-9
- LDE225 Diphosphate
Catalog No.:BCC1693
CAS No.:1218778-77-8
- GKT137831
Catalog No.:BCC5460
CAS No.:1218942-37-0
- Dorsomorphin 2HCl
Catalog No.:BCC4361
CAS No.:1219168-18-9
- (-)-MK 801
Catalog No.:BCC4593
CAS No.:121917-57-5
- CCT 031374 hydrobromide
Catalog No.:BCC6258
CAS No.:1219184-91-4
- Aurothioglucose
Catalog No.:BCC5446
CAS No.:12192-57-3
- Sophoraflavanone C
Catalog No.:BCN3543
CAS No.:121927-91-1
- Dihydrodaidzin
Catalog No.:BCN2879
CAS No.:121927-96-6
Beta Amyloid Differently Modulate Nicotinic and Muscarinic Receptor Subtypes which Stimulate in vitro and in vivo the Release of Glycine in the Rat Hippocampus.[Pubmed:22866037]
Front Pharmacol. 2012 Jul 27;3:146.
Using both in vitro (hippocampal synaptosomes in superfusion) and in vivo (microdialysis) approaches we investigated whether and to what extent beta amyloid peptide 1-40 (Abeta 1-40) interferes with the cholinergic modulation of the release of glycine (GLY) in the rat hippocampus. The nicotine-evoked overflow of endogenous GLY in hippocampal synaptosomes in superfusion was significantly inhibited by Abeta 1-40 (10 nM) while increasing the concentration to 100 nM the inhibitory effect did not further increase. Both the Choline (Ch; alpha7 agonist; 1 mM) and the 5-Iodo-A-85380 dihydrochloride (5IA85380, alpha4beta2 agonist; 10 nM)-evoked GLY overflow were inhibited by Abeta 1-40 at 100 nM but not at 10 nM concentrations. The KCl evoked [(3)H]GLY and [(3)H]Acetylcholine (ACh) overflow were strongly inhibited in presence of oxotremorine; however this inhibitory muscarinic effect was not affected by Abeta 1-40. The effects of Abeta 1-40 on the administration of nicotine, veratridine, 5IA85380, and PHA543613 hydrochloride (PHA543613; a selective agonist of alpha7 subtypes) on hippocampal endogenous GLY release in vivo were also studied. Abeta 1-40 significantly reduced (at 10 muM but not at 1 muM) the nicotine-evoked in vivo release of GLY. Abeta 1-40 (at 10 muM but not at 1 muM) significantly inhibited the PHA543613 (1 mM)-elicited GLY overflow while was ineffective on the GLY overflow evoked by 5IA85380 (1 mM). Abeta 40-1 (10 muM) did not produce any inhibitory effect on nicotine-evoked GLY overflow both in the in vitro and in vivo experiments. Our results indicate that (a) the cholinergic modulation of the release of GLY occurs by the activation of both alpha7 and alpha4beta2 nicotinic ACh receptors (nAChRs) as well as by the activation of inhibitory muscarinic ACh receptors (mAChRs) and (b) Abeta 1-40 can modulate cholinergic evoked GLY release exclusively through the interaction with alpha7 and the alpha4beta2 nAChR nicotinic receptors but not through mAChR subtypes.
Dual effect of beta-amyloid on alpha7 and alpha4beta2 nicotinic receptors controlling the release of glutamate, aspartate and GABA in rat hippocampus.[Pubmed:22253754]
PLoS One. 2012;7(1):e29661.
BACKGROUND: We previously showed that beta-amyloid (Abeta), a peptide considered as relevant to Alzheimer's Disease, is able to act as a neuromodulator affecting neurotransmitter release in absence of evident sign of neurotoxicity in two different rat brain areas. In this paper we focused on the hippocampus, a brain area which is sensitive to Alzheimer's Disease pathology, evaluating the effect of Abeta (at different concentrations) on the neurotransmitter release stimulated by the activation of pre-synaptic cholinergic nicotinic receptors (nAChRs, alpha4beta2 and alpha7 subtypes). Particularly, we focused on some neurotransmitters that are usually involved in learning and memory: glutamate, aspartate and GABA. METHODOLOGY/FINDINGS: WE USED A DUAL APPROACH: in vivo experiments (microdialysis technique on freely moving rats) in parallel to in vitro experiments (isolated nerve endings derived from rat hippocampus). Both in vivo and in vitro the administration of nicotine stimulated an overflow of aspartate, glutamate and GABA. This effect was greatly inhibited by the highest concentrations of Abeta considered (10 microM in vivo and 100 nM in vitro). In vivo administration of 100 nM Abeta (the lowest concentration considered) potentiated the GABA overflow evoked by nicotine. All these effects were specific for Abeta and for nicotinic secretory stimuli. The in vitro administration of either choline or 5-Iodo-A-85380 dihydrochloride (alpha7 and alpha4beta2 nAChRs selective agonists, respectively) elicited the hippocampal release of aspartate, glutamate, and GABA. High Abeta concentrations (100 nM) inhibited the overflow of all three neurotransmitters evoked by both choline and 5-Iodo-A-85380 dihydrochloride. On the contrary, low Abeta concentrations (1 nM and 100 pM) selectively acted on alpha7 subtypes potentiating the choline-induced release of both aspartate and glutamate, but not the one of GABA. CONCLUSIONS/SIGNIFICANCE: The results reinforce the concept that Abeta has relevant neuromodulatory effects, which may span from facilitation to inhibition of stimulated release depending upon the concentration used.
Presynaptic nicotinic alpha7 and non-alpha7 receptors stimulate endogenous GABA release from rat hippocampal synaptosomes through two mechanisms of action.[Pubmed:21346795]
PLoS One. 2011 Feb 8;6(2):e16911.
BACKGROUND: Although converging evidence has suggested that nicotinic acetylcholine receptors (nAChR) play a role in the modulation of GABA release in rat hippocampus, the specific involvement of different nAChR subtypes at presynaptic level is still a matter of debate. In the present work we investigated, using selective alpha7 and alpha4beta2 nAChR agonists, the presence of different nAChR subtypes on hippocampal GABA nerve endings to assess to what extent and through which mechanisms they stimulate endogenous GABA release. METHODOLOGY/FINDINGS: All agonists elicited GABA overflow. Choline (Ch)-evoked GABA overflow was dependent to external Ca(2+), but unaltered in the presence of Cd(2+), tetrodotoxin (TTX), dihydro-beta-erythroidine (DHbetaE) and 1-(4,4-Diphenyl-3-butenyl)-3-piperidinecarboxylic acid hydrochloride SKF 89976A. The effect of Ch was blocked by methyllycaconitine (MLA), alpha-bungarotoxin (alpha-BTX), dantrolene, thapsigargin and xestospongin C, suggesting that GABA release might be triggered by Ca(2+) entry into synaptosomes through the alpha7 nAChR channel with the involvement of calcium from intracellular stores. Additionally, 5-Iodo-A-85380 dihydrochloride (5IA85380) elicited GABA overflow, which was Ca(2+) dependent, blocked by Cd(2+), and significantly inhibited by TTX and DHbetaE, but unaffected by MLA, SKF 89976A, thapsigargin and xestospongin C and dantrolene. These findings confirm the involvement of alpha4beta2 nAChR in 5IA85380-induced GABA release that seems to occur following membrane depolarization and opening calcium channels. CONCLUSIONS/SIGNIFICANCE: Rat hippocampal synaptosomes possess both alpha7 and alpha4beta2 nAChR subtypes, which can modulate GABA release via two distinct mechanisms of action. The finding that GABA release evoked by the mixture of sub-maximal concentration of 5IA85380 plus sub-threshold concentrations of Ch was significantly larger than that elicited by the sum of the effects of the two agonists is compatible with the possibility that they coexist on the same nerve terminals. These findings would provide the basis for possible selective pharmacological strategies to treat neuronal disorders that involve the dysfunction of hippocampal cholinergic system.
Pre-synaptic nicotinic receptors evoke endogenous glutamate and aspartate release from hippocampal synaptosomes by way of distinct coupling mechanisms.[Pubmed:20633015]
Br J Pharmacol. 2010 Nov;161(5):1161-71.
BACKGROUND AND PURPOSE: The present work aimed to investigate whether and through which mechanisms selective alpha7 and alpha4beta2 nicotinic receptor (nAChR) agonists stimulate endogenous glutamate (GLU) and aspartate (ASP) release in rat hippocampus. EXPERIMENTAL APPROACH: Rat hippocampal synaptosomes were purified on Percoll gradients and superfused in vitro to study endogenous GLU and ASP release. The synaptosomes were superfused with selective alpha7 and alpha4beta2 nAChR agonists and antagonists. The excitatory amino acid (EAA) content of the samples of superfusate was determined by HPLC after pre-column derivatization and separation on a chromatographic column coupled with fluorimetric detection. KEY RESULTS: Choline (Ch), a selective alpha7 receptor agonist, elicited a significant release of both GLU and ASP which was blocked by the alpha7 receptor antagonist methyllycaconitine (MLA), but was unaltered by the alpha4beta2 receptor antagonist dihydro-beta-erythroidine (DHbetaE). The stimulant effect of Ch was strongly reduced in a Ca(2+) -free medium, was not inhibited by Cd(2+) and tetrodotoxin (TTX), but was antagonized by dantrolene, xestospongin C and thapsigargin. 5-Iodo-A-85380 dihydrochloride (5IA85380), a selective alpha4beta2 receptor agonist, elicited EAA release in a DHbetaE-sensitive, MLA-insensitive fashion. The 5IA85380-evoked release was dependent on extracellular Ca(2+) , blocked by Cd(2+) and TTX, but unaffected by dantrolene. CONCLUSIONS AND IMPLICATIONS: Our study shows for the first time that rat hippocampal synaptosomes possess alpha7 and alpha4beta2 nAChR subtypes, which can enhance the release of endogenous GLU and ASP via two distinct mechanisms of action. These results extend our knowledge of the nicotinic modulation of excitatory synaptic transmission in the hippocampus.
Functional responses and subunit composition of presynaptic nicotinic receptor subtypes explored using the novel agonist 5-iodo-A-85380.[Pubmed:15527819]
Neuropharmacology. 2004 Nov;47(6):848-59.
The novel compound 5-iodo-A-85380 binds with higher affinity to alpha4beta2* nicotinic acetylcholine receptors (nAChR), compared with other nAChR subtypes (Mukhin et al., 2000). In the present study, we have confirmed that in competition binding assays for three major nAChR subtypes, 5-iodo-A-85380 is 850 and 27,000-fold more potent at rat brain alpha4beta2* binding sites than at alpha3beta4 and alpha7 subtypes, respectively. In functional assays, 5-iodo-A-85380 potently activated (EC50 12-35 nM) both alpha-CTx-MII-sensitive and -insensitive components of [3H]dopamine release from rat striatal synaptosomes, corresponding to alpha6beta2* and alpha4beta2* nAChR, respectively. 5-Iodo-A-85380 was markedly less potent at eliciting [3H]ACh release from rat interpeduncular nucleus synaptosomes, [3H]noradrenaline release from rat hippocampal slices, and Ca2+ increases in a cell line expressing rat alpha3beta4 nAChR (EC50 = 5, 3.2, 1.6 microM, respectively). As predicted by ligand binding studies, 5-iodo-A-85380 is a more discriminating agonist than the parent compound epibatidine. However, it is not specific for alpha4beta2* nAChR as it also potently activates alpha6beta2* nAChR.
5-Iodo-A-85380, an alpha4beta2 subtype-selective ligand for nicotinic acetylcholine receptors.[Pubmed:10692507]
Mol Pharmacol. 2000 Mar;57(3):642-9.
In an effort to develop selective radioligands for in vivo imaging of neuronal nicotinic acetylcholine receptors (nAChRs), we synthesized 5-iodo-3-(2(S)-azetidinylmethoxy)pyridine (5-iodo-A-85380) and labeled it with (125)I and (123)I. Here we present the results of experiments characterizing this radioiodinated ligand in vitro. The affinity of 5-[(125)I]iodo-A-85380 for alpha4beta2 nAChRs in rat and human brain is defined by K(d) values of 10 and 12 pM, respectively, similar to that of epibatidine (8 pM). In contrast to epibatidine, however, 5-iodo-A-85380 is more selective in binding to the alpha4beta2 subtype than to other nAChR subtypes. In rat adrenal glands, 5-iodo-A-85380 binds to nAChRs containing alpha3 and beta4 subunits with 1/1000th the affinity of epibatidine, and exhibits 1/60th and 1/190th the affinity of epibatidine at alpha7 and muscle-type nAChRs, respectively. Moreover, unlike epibatidine and cytisine, 5-[(125)I]iodo-A-85380 shows no binding in any brain regions in mice homozygous for a mutation in the beta2 subunit of nAChRs. Binding of 5-[(125)I]iodo-A-85380 in rat brain is reversible, and is characterized by high specificity and a slow rate of dissociation of the receptor-ligand complex (t(1/2) for dissociation approximately 2 h). These properties, along with other features observed previously in in vivo experiments (low toxicity, rapid penetration of the blood-brain barrier, and a high ratio of specific to nonspecific binding), suggest that this compound, labeled with (125)I or (123)I, is superior to other radioligands available for in vitro and in vivo studies of alpha4beta2 nAChRs, respectively.
2-, 5-, and 6-Halo-3-(2(S)-azetidinylmethoxy)pyridines: synthesis, affinity for nicotinic acetylcholine receptors, and molecular modeling.[Pubmed:9733494]
J Med Chem. 1998 Sep 10;41(19):3690-8.
3-(2(S)-Azetidinylmethoxy)pyridine (A-85380) has been identified recently as a ligand with high affinity for nicotinic acetylcholine receptors (nAChRs). Here we report the synthesis and in vitro nAChR binding of a series of 10 pyridine-modified analogues of A-85380. The novel compounds feature a halogen substituent at position 2, 5, or 6 of the 3-pyridyl fragment. Those with the substituents at position 5 or 6, as well as the 2-fluoro analogue, possess subnanomolar affinity for nAChRs in membranes from rat brain. For these ligands, Ki values range from 11 to 210 pM, as measured by competition with (+/-)-[3H]epibatidine. In contrast, 2-chloro, 2-bromo, and 2-iodo analogues exhibit substantially lower affinity. AM1 quantum chemical calculations demonstrate that the bulky substituents at position 2 cause notable changes in the molecular geometry. The high-affinity members of the series and (+)-epibatidine display a tight fit superposition of low-energy stable conformers. The new ligands with high affinity for nAChRs may be of interest as pharmacological probes, potential medications, and candidates for developing radiohalogenated tracers to study nAChRs.


