4-ChlorodehydromethyltestosteroneCAS# 2446-23-3 |
Quality Control & MSDS
Number of papers citing our products

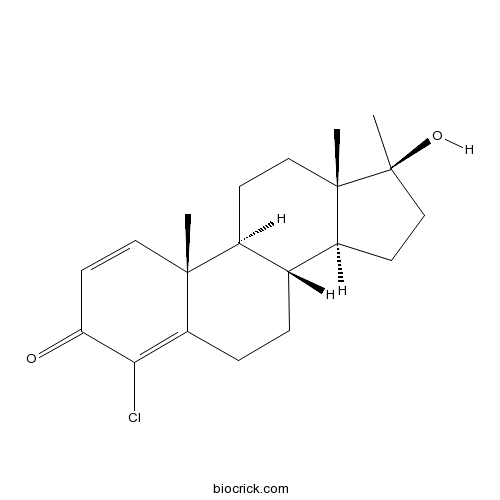
Chemical structure

3D structure
| Cas No. | 2446-23-3 | SDF | Download SDF |
| PubChem ID | 98521 | Appearance | Powder |
| Formula | C20H27ClO2 | M.Wt | 334.9 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (8R,9S,10R,13S,14S,17S)-4-chloro-17-hydroxy-10,13,17-trimethyl-7,8,9,11,12,14,15,16-octahydro-6H-cyclopenta[a]phenanthren-3-one | ||
| SMILES | CC12CCC3C(C1CCC2(C)O)CCC4=C(C(=O)C=CC34C)Cl | ||
| Standard InChIKey | AGUNEISBPXQOPA-XMUHMHRVSA-N | ||
| Standard InChI | InChI=1S/C20H27ClO2/c1-18-9-8-16(22)17(21)15(18)5-4-12-13(18)6-10-19(2)14(12)7-11-20(19,3)23/h8-9,12-14,23H,4-7,10-11H2,1-3H3/t12-,13+,14+,18-,19+,20+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

4-Chlorodehydromethyltestosterone Dilution Calculator

4-Chlorodehydromethyltestosterone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.986 mL | 14.9298 mL | 29.8597 mL | 59.7193 mL | 74.6491 mL |
| 5 mM | 0.5972 mL | 2.986 mL | 5.9719 mL | 11.9439 mL | 14.9298 mL |
| 10 mM | 0.2986 mL | 1.493 mL | 2.986 mL | 5.9719 mL | 7.4649 mL |
| 50 mM | 0.0597 mL | 0.2986 mL | 0.5972 mL | 1.1944 mL | 1.493 mL |
| 100 mM | 0.0299 mL | 0.1493 mL | 0.2986 mL | 0.5972 mL | 0.7465 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Daphnetin dimethyl ether
Catalog No.:BCN2735
CAS No.:2445-80-9
- Nonivamide
Catalog No.:BCN2325
CAS No.:2444-46-4
- 3,5,7,15-Tetraacetoxy-9-nicotinoyloxy-6(17),11-jatrophadien-14-one
Catalog No.:BCN6592
CAS No.:244277-75-6
- LFM-A13
Catalog No.:BCC6472
CAS No.:244240-24-2
- JTC-801
Catalog No.:BCC3800
CAS No.:244218-51-7
- Celaphanol A
Catalog No.:BCN5101
CAS No.:244204-40-8
- Pulchinenoside E2
Catalog No.:BCN8186
CAS No.:244202-36-6
- L-748,337
Catalog No.:BCC7475
CAS No.:244192-94-7
- Taxumairol R
Catalog No.:BCN6939
CAS No.:244167-04-2
- L-798,106
Catalog No.:BCC7654
CAS No.:244101-02-8
- Beta-Rotunol
Catalog No.:BCN6628
CAS No.:24405-57-0
- (+)-Epipinoresinol
Catalog No.:BCN3255
CAS No.:24404-50-0
- Sanguinarine
Catalog No.:BCN5102
CAS No.:2447-54-3
- Sulfadoxine
Catalog No.:BCC4726
CAS No.:2447-57-6
- Pseudoakuammigine
Catalog No.:BCN4812
CAS No.:2447-70-3
- Dapivirine (TMC120)
Catalog No.:BCC3882
CAS No.:244767-67-7
- Z-D-Phe-OH
Catalog No.:BCC2755
CAS No.:2448-45-5
- Bryonolic acid
Catalog No.:BCN5103
CAS No.:24480-45-3
- Isochlorogenic acid A
Catalog No.:BCN5908
CAS No.:2450-53-5
- Acetylshikonin
Catalog No.:BCN2665
CAS No.:24502-78-1
- Dimethylacrylshikonin
Catalog No.:BCN2310
CAS No.:23444-70-4
- 19-Oxocinobufagin
Catalog No.:BCN8229
CAS No.:24512-59-2
- 19-Oxocinobufotalin
Catalog No.:BCN8233
CAS No.:24512-60-5
- Gardenoside
Catalog No.:BCN2383
CAS No.:24512-62-7
Screening of free 17-alkyl-substituted anabolic steroids in human urine by liquid chromatography-electrospray ionization tandem mass spectrometry.[Pubmed:15013688]
Steroids. 2004 Feb;69(2):101-9.
A qualitative liquid chromatography-electrospray ionization tandem mass spectrometry method was developed for screening of the abuse of 4-Chlorodehydromethyltestosterone, danazol, fluoxymesterone, formebolone, metandienone, oxandrolone, and stanozolol. The introduced method measures simultaneously nine different 17-alkyl-substituted anabolic androgenic steroids or their unconjugated metabolites in human urine, using methyltestosterone as an internal standard. Sample preparation involved one-step liquid extraction. Liquid chromatographic separation was achieved on a reversed-phase column with methanol-water gradient containing 5 mmol/l ammonium acetate and 0.01% (v/v) acetic acid. Compounds were ionized in the positive mode and detected by multiple reaction monitoring. All steroids within the study could be selectively detected in urine with detection limits of 0.1-2.0 ng/ml. The method showed good linearity up to 250 ng/ml with correlation coefficients higher than 0.9947. With simple and fast sample preparation, low limits of detection, and high selectivity and precision, the developed method provides advantages over the present testing methods and has the potential for routine qualitative screening method of unconjugated 17-alkyl-substituted anabolic steroids in human urine.
17-Epimerization of 17 alpha-methyl anabolic steroids in humans: metabolism and synthesis of 17 alpha-hydroxy-17 beta-methyl steroids.[Pubmed:1448813]
Steroids. 1992 Nov;57(11):537-50.
The 17-epimers of the anabolic steroids bolasterone (I), 4-Chlorodehydromethyltestosterone (II), fluoxymesterone (III), furazabol (IV), metandienone (V), mestanolone (VI), methyltestosterone (VII), methandriol (VIII), oxandrolone (IX), oxymesterone (X), oxymetholone (XI), stanozolol (XII), and the human metabolites 7 alpha,17 alpha-dimethyl-5 beta-androstane-3 alpha,17 beta-diol (XIII) (metabolite of I), 6 beta-hydroxymetandienone (XIV) (metabolite of V), 17 alpha-methyl-5 beta-androst-1-ene-3 alpha,17 beta-diol (XV) (metabolite of V), 3'-hydroxystanozolol (XVI) (metabolite of XII), as well as the reference substances 17 beta-hydroxy-17 alpha-methyl-5 beta-androstan-3-one (XVII), 17 beta-hydroxy-17 alpha-methyl-5 beta-androst-1-en-3-one (XVIII) (also a metabolite of V), the four isomers 17 alpha-methyl-5 alpha-androstane-3 alpha,17 beta-diol (XIX) (also a metabolite of VI, VII, and XI), 17 alpha-methyl-5 alpha-androstane-3 beta,17 beta-diol (XX), 17 alpha-methyl-5 beta-androstane-3 alpha,17 beta-diol (XXI) (also a metabolite of V, VII, and VIII), 17 alpha-methyl-5 beta-androstane-3 beta,17 beta-diol (XXII), and 17 beta-hydroxy-7 alpha,17 alpha-dimethyl-5 beta-androstan-3-one (XXIII) were synthesized via a 17 beta-sulfate that spontaneously hydrolyzed in water to several dehydration products, and to the 17 alpha-hydroxy-17 beta-methyl epimer. The 17 beta-sulfate was prepared by reaction of the 17 beta-hydroxy-17 alpha-methyl steroid with sulfur trioxide pyridine complex. The 17 beta-methyl epimers are eluted in gas chromatography as trimethylsilyl derivatives from a capillary SE-54 or OV-1 column 70-170 methylen units before the corresponding 17 alpha-methyl epimer. The electron impact mass spectra of the underivatized and trimethylsilylated epimers are in most cases identical and only for I, II, and V was a differentiation between the 17-epimers possible. 1H nuclear magnetic resonance (NMR) spectra show for the 17 beta-methyl epimer a chemical shift for the C-18 protons (singlet) of about 0.175 ppm (in deuterochloroform) to a lower field. 13C NMR spectra display differences for the 17-epimeric steroids in shielding effects for carbons 12-18 and 20. Excretion studies with I-XII with identification and quantification of 17-epimeric metabolites indicate that the extent of 17-epimerization depends on the A-ring structure and shows a great variation for the different 17 alpha-methyl anabolic steroids.


