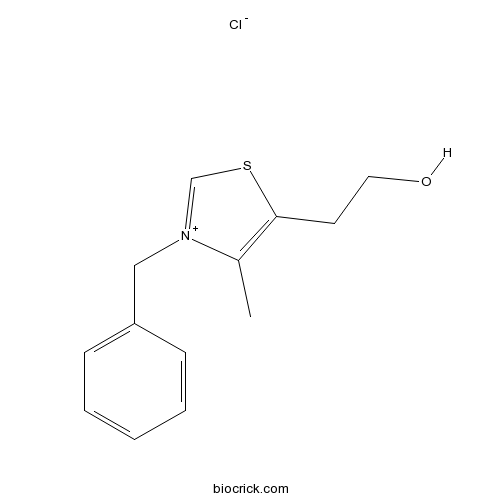
3-Benzyl-5-(2-hydroxyethyl)-4-methylthiazolium chlorideCAS# 4568-71-2 |
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 4568-71-2 | SDF | Download SDF |
| PubChem ID | 2833352 | Appearance | Powder |
| Formula | C13H16ClNOS | M.Wt | 269.8 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 2-(3-benzyl-4-methyl-1,3-thiazol-3-ium-5-yl)ethanol;chloride | ||
| SMILES | CC1=C(SC=[N+]1CC2=CC=CC=C2)CCO.[Cl-] | ||
| Standard InChIKey | IWSVLBKHBJGMAA-UHFFFAOYSA-M | ||
| Standard InChI | InChI=1S/C13H16NOS.ClH/c1-11-13(7-8-15)16-10-14(11)9-12-5-3-2-4-6-12;/h2-6,10,15H,7-9H2,1H3;1H/q+1;/p-1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

3-Benzyl-5-(2-hydroxyethyl)-4-methylthiazolium chloride Dilution Calculator

3-Benzyl-5-(2-hydroxyethyl)-4-methylthiazolium chloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.7064 mL | 18.5322 mL | 37.0645 mL | 74.129 mL | 92.6612 mL |
| 5 mM | 0.7413 mL | 3.7064 mL | 7.4129 mL | 14.8258 mL | 18.5322 mL |
| 10 mM | 0.3706 mL | 1.8532 mL | 3.7064 mL | 7.4129 mL | 9.2661 mL |
| 50 mM | 0.0741 mL | 0.3706 mL | 0.7413 mL | 1.4826 mL | 1.8532 mL |
| 100 mM | 0.0371 mL | 0.1853 mL | 0.3706 mL | 0.7413 mL | 0.9266 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Neocnidilide
Catalog No.:BCN8174
CAS No.:4567-33-3
- H-Glu(OBzl)-OBzl.HCl
Catalog No.:BCC2927
CAS No.:4561-10-8
- Zaurategrast
Catalog No.:BCC2070
CAS No.:455264-31-0
- Isovouacapenol C
Catalog No.:BCN6557
CAS No.:455255-15-9
- 4-Benzoyl-3-methyl-1-phenyl-5-pyrazolone
Catalog No.:BCC8695
CAS No.:4551-69-3
- Grossamide K
Catalog No.:BCC4547
CAS No.:
- Corosolic acid
Catalog No.:BCN5503
CAS No.:4547-24-4
- Acetophenone tosylhydrazone
Catalog No.:BCC8804
CAS No.:4545-21-5
- kobe2602
Catalog No.:BCC5291
CAS No.:454453-49-7
- Nudifloside D
Catalog No.:BCN7005
CAS No.:454212-54-5
- PSB 11 hydrochloride
Catalog No.:BCC7239
CAS No.:453591-58-7
- Motesanib
Catalog No.:BCC1776
CAS No.:453562-69-1
- 5-O-Methylgenistein
Catalog No.:BCN7714
CAS No.:4569-98-6
- 1alpha, 24, 25-Trihydroxy VD2
Catalog No.:BCC1298
CAS No.:457048-34-9
- Pyridone 6
Catalog No.:BCC1874
CAS No.:457081-03-7
- PA 452
Catalog No.:BCC8005
CAS No.:457657-34-0
- BMS 470539 dihydrochloride
Catalog No.:BCC7850
CAS No.:457893-92-4
-
Scutebarbatine L
Catalog No.:BCN8369
CAS No.:960302-91-4
- Curcumin
Catalog No.:BCN5504
CAS No.:458-37-7
- Ilicic acid
Catalog No.:BCN5505
CAS No.:4586-68-9
- Boc-Asn-ONp
Catalog No.:BCC3072
CAS No.:4587-33-1
- SW033291
Catalog No.:BCC3981
CAS No.:459147-39-8
- JNJ-7777120
Catalog No.:BCC4543
CAS No.:459168-41-3
- SB 452533
Catalog No.:BCC7620
CAS No.:459429-39-1
Inhibition of mammalian carbonic anhydrase isoforms I, II and VI with thiamine and thiamine-like molecules.[Pubmed:22145674]
J Enzyme Inhib Med Chem. 2013 Apr;28(2):316-9.
Here we determined the in vitro inhibitory effects of 5-(2-hydroxyethyl)-3,4-dimethylthiazolium iodide (1), 3-Benzyl-5-(2-hydroxyethyl)-4-methylthiazolium chloride (2) and thiamine (3) on human erythrocyte carbonic anhydrase I, II isozymes (hCA I and hCA II) and secreted isoenzyme CA VI. K(I) values ranged from 0.38 to 2.27 microM for hCA I, 0.085 to 0.784 microM for hCA II and 0.062 to 0.593 microM for hCA VI, respectively. The compounds displayed relatively strong actions on hCA II, in the same range as the clinically used sulfonamidesethoxzolamide, zonisamide and acetazolamide.


