3-Amino-5-mercapto-1,2,4-triazoleCAS# 16691-43-3 |
Quality Control & MSDS
Number of papers citing our products

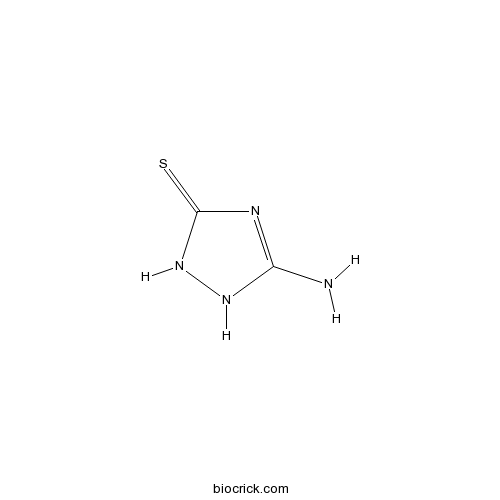
Chemical structure

3D structure
| Cas No. | 16691-43-3 | SDF | Download SDF |
| PubChem ID | 2723869 | Appearance | Powder |
| Formula | C2H4N4S | M.Wt | 116 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 5-amino-1,2-dihydro-1,2,4-triazole-3-thione | ||
| SMILES | C1(=NC(=S)NN1)N | ||
| Standard InChIKey | WZUUZPAYWFIBDF-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C2H4N4S/c3-1-4-2(7)6-5-1/h(H4,3,4,5,6,7) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

3-Amino-5-mercapto-1,2,4-triazole Dilution Calculator

3-Amino-5-mercapto-1,2,4-triazole Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 8.6207 mL | 43.1034 mL | 86.2069 mL | 172.4138 mL | 215.5172 mL |
| 5 mM | 1.7241 mL | 8.6207 mL | 17.2414 mL | 34.4828 mL | 43.1034 mL |
| 10 mM | 0.8621 mL | 4.3103 mL | 8.6207 mL | 17.2414 mL | 21.5517 mL |
| 50 mM | 0.1724 mL | 0.8621 mL | 1.7241 mL | 3.4483 mL | 4.3103 mL |
| 100 mM | 0.0862 mL | 0.431 mL | 0.8621 mL | 1.7241 mL | 2.1552 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- A-1210477
Catalog No.:BCC6508
CAS No.:1668553-26-1
- Chebulanin
Catalog No.:BCN3261
CAS No.:166833-80-3
- H-D-Orn-OH. HCl
Catalog No.:BCC3004
CAS No.:16682-12-5
- H-Gly-NH2.HCl
Catalog No.:BCC2947
CAS No.:1668-10-6
- Desmopressin
Catalog No.:BCC1525
CAS No.:16679-58-6
- Prerubialatin
Catalog No.:BCN6895
CAS No.:1667718-89-9
- Z-Tyr(Bzl)-OH
Catalog No.:BCC2735
CAS No.:16677-29-5
- Naltrexone HCl
Catalog No.:BCC4613
CAS No.:16676-29-2
- H-Cys(Trt)-NH2
Catalog No.:BCC2912
CAS No.:166737-85-5
- cis-Mulberroside A
Catalog No.:BCN3911
CAS No.:166734-06-1
- 4,4'-Bis(chloromethyl)biphenyl
Catalog No.:BCC8658
CAS No.:1667-10-3
- Anidulafungin
Catalog No.:BCC4233
CAS No.:166663-25-8
- Tetramethylkaempferol
Catalog No.:BCN8082
CAS No.:16692-52-7
- N-Acetoacetylmorpholine
Catalog No.:BCC9078
CAS No.:16695-54-8
- BMS 453
Catalog No.:BCC7679
CAS No.:166977-43-1
- Fmoc-D-Cys(Trt)-OH
Catalog No.:BCC3481
CAS No.:167015-11-4
- Pitavastatin ethyl ester
Catalog No.:BCC9122
CAS No.:167073-19-0
- 10-Oxo Docetaxel
Catalog No.:BCC5409
CAS No.:167074-97-7
- Autocamtide-2-related inhibitory peptide
Catalog No.:BCC7153
CAS No.:167114-91-2
- R-96544 hydrochloride
Catalog No.:BCC7164
CAS No.:167144-80-1
- Furagin
Catalog No.:BCC1582
CAS No.:1672-88-4
- Clevidipine Butyrate
Catalog No.:BCC4401
CAS No.:167221-71-8
- Cyanidin 3-Sophoroside-5-Glucoside
Catalog No.:BCC8158
CAS No.:16727-02-9
- Malvidin 3,5-Diglucoside
Catalog No.:BCC8206
CAS No.:16727-30-3
Advanced oxygen reduction reaction catalyst based on nitrogen and sulfur co-doped graphene in alkaline medium.[Pubmed:25255312]
Phys Chem Chem Phys. 2014 Nov 14;16(42):23196-205.
A novel nitrogen and sulfur co-doped graphene (N-S-G) catalyst for oxygen reduction reaction (ORR) has been prepared by pyrolysing graphite oxide and poly[3-Amino-5-mercapto-1,2,4-triazole] composite (PAMTa). The atomic percentage of nitrogen and sulfur for the prepared N-S-G can be adjusted by controlling the pyrolysis temperature. Furthermore, the catalyst pyrolysed at 1000 degrees C, denoted N-S-G 1000, exhibits the highest catalytic activity for ORR, which displays the highest content of graphitic-N and thiophene-S among all the pyrolysed samples. The electrocatalytic performance of N-S-G 1000 is significantly better than that of PAMTa and reduced graphite oxide composite. Remarkably, the N-S-G 1000 catalyst is comparable with Pt/C in terms of the onset and half-wave potentials, and displays larger kinetic limiting current density and better methanol tolerance and stability than Pt/C for ORR in an alkaline medium.
A SERS-based pH sensor utilizing 3-amino-5-mercapto-1,2,4-triazole functionalized Ag nanoparticles.[Pubmed:24409451]
Analyst. 2014 Mar 7;139(5):1101-11.
We report the first use of 3-Amino-5-mercapto-1,2,4-triazole (AMT) to construct a surface-enhanced Raman scattering (SERS) based pH nano- and microsensor, utilizing silver nanoparticles. We optimize the procedure of homogenous attachment of colloidal silver to micrometer-sized silica beads via an aminosilane linker. Such micro-carriers are potential optically trappable SERS microprobes. It is demonstrated that the SERS spectrum of AMT is strongly dependent on the pH of the surroundings, as the transformation between two different adsorption modes, upright (A form) and lying flat (B form) orientation, is provoked by pH variation. The possibility of tuning the nanosensor working range by changing the concentration of AMT in the surrounding solution is demonstrated. A strong correlation between the pH response of the nanosensor and the AMT concentration in solution is found to be controlled by the interactions between the surface and solution molecules. In the absence of the AMT monomer, the performance of both the nano- and microsensor is shifted substantially to the strongly acidic pH range, from 1.5 to 2.5 and from 1.0 to 2.0, respectively, which is quite unique even for SERS-based sensors.
Combined repeated-dose and reproductive/developmental toxicity screening test of 3-amino-5-mercapto-1,2,4-triazole in rats.[Pubmed:24067724]
J Toxicol Sci. 2013;38(5):759-73.
The substance 3-Amino-5-mercapto-1,2,4-triazole (AMT, CAS No. 16691-43-3) was daily administered by gavage to Crl:CD (SD)IGS rats at doses of 0 (control), 10, 50, and 250 mg/kg bw/day. Males (12/group) were treated for a total of 42 days beginning 14 days before mating. Females (12/group) were treated beginning 14 days before mating to day 4 of lactation throughout the mating and gestation periods. No deaths occurred in males but three females died on day 23 of gestation at 250 mg/kg/day. Only temporary decreases in body weight and food intake were found in both sexes at 250 mg/kg/day. There were no considerable changes in general appearance, the functional battery tests, biochemical analysis or urinalysis. Anemia was observed in both sexes at 250 mg/kg/day. The relative weight of thyroid glands was significantly increased in both sexes at 250 mg/kg/day and hypertrophy of thyroid follicular cells was observed in 50 and 250 mg/kg/day males and 250 mg/kg/day females. As this effect on thyroid glands was considered to be the major toxicity, the possible mechanism was discussed comparing with the toxicity of structural similar analogs. Other histopathological changes in males were hypertrophy of centrilobular hepatocytes at 250 mg/kg/day, and anterior pituitary glands at 50 mg/kg/day and more. Vacuolization in renal tubular epithelium of females was observed at 50 and 250 mg/kg/day. For reproduction, the gestation period was prolonged and the delivery index was decreased at 250 mg/kg/day. The number of pups born and the birth index were also reduced. It was thus concluded that the NOAEL for repeated-dose toxicity was 10 mg/kg/day based on the thyrotoxicity and renal toxicity, and that the NOAEL for reproductive/developmental toxicity was 50 mg/kg/day based on the reduced number of offspring, etc.
Picomolar melamine enhanced the fluorescence of gold nanoparticles: spectrofluorimetric determination of melamine in milk and infant formulas using functionalized triazole capped gold nanoparticles.[Pubmed:23208097]
Biosens Bioelectron. 2013 Apr 15;42:267-72.
We wish to report a simple and sensitive method to determine the melamine in milk and infant formulas using 3-Amino-5-mercapto-1,2,4-triazole capped gold nanoparticles (AMTr-AuNPs) as fluorophore. The AMTr-AuNPs were synthesized by a wet chemical method and were characterized by high-resolution transmission electron microscopy (HR-TEM), and X-ray diffraction, UV-visible and fluorescence spectroscopic techniques. The AMTr-AuNPs show the absorption maximum at 520 nm and emission maximum at 759 nm (lambda(ex)=520 nm). While adding 10 muM melamine, the wine red color of AMTr-AuNPs was changed into purple and the absorption band at 520 nm was decreased. The observed changes were ascribed to the hydrogen bonding interaction between melamine and AMTr-AuNPs, which led to the aggregation of the nanoparticles. This was confirmed by dynamic light scattering and HR-TEM measurements. No appreciable absorption change was observed for AMTr-AuNPs in the presence of less than micromolar concentrations of melamine. But, the emission intensity of AMTr-AuNPs was enhanced even in the presence of picomolar concentration of melamine. Based on the enhancement of emission intensity, the concentration of melamine was determined. The present fluorophore showed an extreme selectivity towards the determination of 100 nM melamine in the presence of 500-fold common interferents. The good linearly was observed from 1x10(-)(9) to 100x10(-)(1)(2) M melamine and a detection limit was found to be 10 fM/L (S/N=3). The proposed method was successfully applied to determine melamine in cow milk and infant formulas. The obtained results were validated with HPLC.
Highly sensitive determination of uric acid in the presence of major interferents using a conducting polymer film modified electrode.[Pubmed:22763421]
Bioelectrochemistry. 2012 Dec;88:22-9.
This paper describes the sensitive and selective determination of uric acid (UA) in the presence of important interferences, ascorbic acid (AA), dopamine (DA), tyrosine (Tyr) and methionine (Met) at physiological pH using an electropolymerized film of 3-Amino-5-mercapto-1,2,4-triazole on glassy carbon (p-AMTa) electrode. The p-AMTa electrode shows an excellent electrocatalytic activity towards UA. This was understood from the observed higher oxidation current and heterogeneous rate constant (3.24x10(-5)ms(-1)) for UA when compared to bare GC electrode (4.63x10(-6)ms(-1)). The selective determination of UA in the presence of 1000-fold excess of AA was achieved using p-AMTa electrode. Further, the p-AMTa electrode was successfully used for the simultaneous and selective determination of UA in the presence of important interferences, DA, Tyr and Met. Using amperometric method, 40nM UA was detected for the first time. The current response of UA was increased linearly while increasing its concentration from 40nM to 0.1mM and a detection limit was found to be 0.52nM (S/N=3). Finally, the practical application of the present method was demonstrated by determining UA in human urine and blood serum samples.
Selective determination of inosine in the presence of uric acid and hypoxanthine using modified electrode.[Pubmed:22080039]
Anal Biochem. 2012 Feb 1;421(1):278-84.
This article describes the selective determination of inosine (INO) in the presence of important physiological interferents, uric acid (UA) and hypoxanthine (HXN), by differential pulse voltammetry at physiological pH (7.2) using the electropolymerized film of 3-Amino-5-mercapto-1,2,4-triazole (p-AMTa) modified glassy carbon (GC) electrode. The electropolymerization of AMTa was carried out by the potentiodynamic method in 0.1M H(2)SO(4). An atomic force microscopy image shows that the p-AMTa film contains a spherical-like structure. Bare GC electrode fails to resolve the voltammetric signal of INO in the presence of UA and HXN due to the surface fouling caused by the oxidized products of UA and HXN. However, p-AMTa film modified GC electrode (p-AMTa electrode) not only separates the voltammetric signals of UA, HXN, and INO, with potential differences of 730 mV between UA and HXN and 310 mV between HXN and INO, but also shows enhanced oxidation current for them. The selective determination of INO in the presence of UA and HXN at physiological pH was achieved for the first time. Using the amperometric method, we achieved the lowest detection of 50 nM for INO. The practical application of the current modified electrode was demonstrated by determining the concentration of INO in human blood serum and urine samples.
FT-IR, FT-Raman, ab initio and DFT studies, HOMO-LUMO and NBO analysis of 3-amino-5-mercapto-1,2,4-triazole.[Pubmed:22070998]
Spectrochim Acta A Mol Biomol Spectrosc. 2012 Feb;86:242-51.
The molecular vibrations of 3-Amino-5-mercapto-1,2,4-triazole (AMT) have been investigated in polycrystalline sample, at room temperature, by Fourier transform infrared (FT-IR) and FT-Raman spectroscopies. A detailed vibrational spectral analysis has been carried out and assignments of the fundamental modes have been proposed on the basis of peak positions and relative frequencies, atomic charges, HOMO-LUMO energies and several thermodynamic properties in the ground state were calculated using ab initio Hartree-Fock (HF) and Density Functional Theory, (B3LYP) with 6-311G(d,p) and 6-311++G(d,p) basis sets. With the aid of scaling procedures, observed wave numbers in FT-IR and FT-Raman spectra were analyzed and assigned to different normal modes of the molecule. Most of the modes have wave numbers in the expected range. The theoretical IR and Raman spectra have also been constructed. Natural Bond Orbital (NBO) study explains charge delocalization of the molecule.
Electrochemical sensor for neurotransmitters at physiological pH using a heterocyclic conducting polymer modified electrode.[Pubmed:22048066]
Analyst. 2012 Jan 7;137(1):209-15.
We report the simultaneous determination of two neurotransmitters, norepinephrine (NEP) and serotonin (5-HT), at physiological pH using the electropolymerized film of 3-Amino-5-mercapto-1,2,4-triazole modified glassy carbon (p-AMTa) electrode. A bare glassy carbon (GC) electrode fails to resolve the voltammetric signals of NEP and 5-HT due to the surface fouling caused by the oxidized products of them. However, the p-AMTa electrode not only separates the voltammetric signals of NEP and 5-HT with a potential difference of 150 mV between NEP and 5-HT but also shows higher oxidation currents for them. The simultaneous determination of NEP and 5-HT was successfully achieved at p-AMTa electrode using differential pulse voltammetry method. The amperometric current response increased linearly with increasing NEP and 5-HT concentration in the range of 1.0 x 10(-8) to 1.0 x 10(-4) M and 1.0 x 10(-8) to 5.0 x 10(-5) M, respectively, and the detection limit was found to be 1.65 x 10(-11) for NEP and 1.32 x 10(-11) M for 5-HT (S/N = 3). The p-AMTa electrode shows better recovery results for spiked NEP and 5-HT in human blood plasma samples.
Au-nanoclusters incorporated 3-amino-5-mercapto-1,2,4-triazole film modified electrode for the simultaneous determination of ascorbic acid, dopamine, uric acid and nitrite.[Pubmed:21982642]
Biosens Bioelectron. 2011 Dec 15;30(1):315-9.
A novel biosensor has been constructed by the electrodeposition of Au-nanoclusters (nano-Au) on poly(3-Amino-5-mercapto-1,2,4-triazole) (p-TA) film modified glassy carbon electrode (GCE) and employed for the simultaneous determination of dopamine (DA), ascorbic acid (AA), uric acid (UA) and nitrite (NO(2)(-)). NH(2) and SH groups exposed to the p-TA layer are helpful for the electrodeposition of nano-Au. The combination of nano-Au and p-TA endow the biosensor with large surface area, good biological compatibility, electricity and stability, high selectivity and sensitivity and flexible and controllable electrodeposition process. In the fourfold co-existence system, the linear calibration plots for AA, DA, UA and NO(2)(-) were obtained over the range of 2.1-50.1 muM, 0.6-340.0 muM, 1.6-110.0 muM and 15.9-277.0 muM with detection limits of 1.1x10(-6) M, 5.0x10(-8) M, 8.0x10(-8) M and 8.9x10(-7) M, respectively. In addition, the modified biosensor was applied to the determination of AA, DA, UA and NO(2)(-) in urine and serum samples by using standard adding method with satisfactory results.
Oxidatively generated DNA damage induced by 3-amino-5-mercapto-1,2,4-triazole, a metabolite of carcinogenic amitrole.[Pubmed:20732334]
Mutat Res. 2010 Dec 10;694(1-2):7-12.
Amitrole (3-amino-1,2,4-triazole) is a widely used herbicide. Amitrole induces thyroid and liver tumors in rodents. However, the mechanism of carcinogenesis by amitrole remains to be clarified. To clarify the mechanism of carcinogenesis induced by amitrole, we investigated the formation of 8-oxo-7,8-dihydro-2'-deoxyguanosine (8-oxodG), a characteristic of oxidatively generated DNA damage, by an amitrole metabolite, 3-Amino-5-mercapto-1,2,4-triazole (AMT), in the presence of Cu(II). The amount of 8-oxodG was increased by AMT in the presence of Cu(II). AMT-induced 8-oxodG formation was enhanced in deuterium oxide (D(2)O), which prolongs the half life of singlet oxygen ((1)O(2)), more than that in H(2)O. Sodium azide and 1,4-diazabicyclo[2,2,2]-octane (DABCO), potent and relatively specific scavengers of (1)O(2), inhibited AMT-mediated 8-oxodG formation. Bathocuproine, a Cu(I) chelator, also inhibited the 8-oxodG formation. On the other hand, typical OH scavengers did not inhibit the generation of 8-oxodG. AMT plus Cu(II) also induced piperidine-labile DNA lesions frequently at every guanine residue. These results suggest that (1)O(2) and Cu(I) play an important role in DNA damage induced by AMT. It is concluded that oxidatively generated DNA damage induced by AMT via the generation of (1)O(2) may contribute to carcinogenicity of amitrole.


