16-O-MethylcafestolCAS# 108214-28-4 |
Quality Control & MSDS
Number of papers citing our products

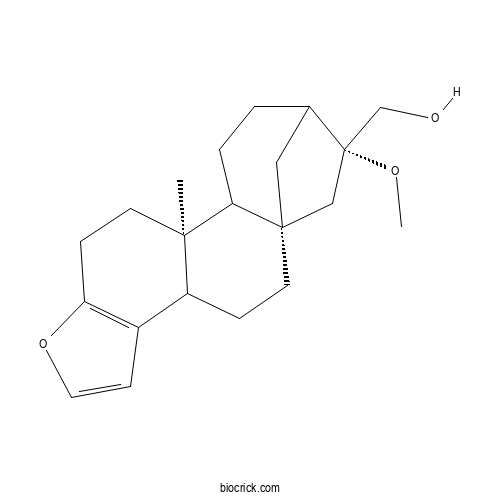
Chemical structure

3D structure
| Cas No. | 108214-28-4 | SDF | Download SDF |
| PubChem ID | 146156386 | Appearance | White powder |
| Formula | C21H30O3 | M.Wt | 330.5 |
| Type of Compound | Isoprenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in chloroform | ||
| Chemical Name | [(1S,12S,17R)-17-methoxy-12-methyl-8-oxapentacyclo[14.2.1.01,13.04,12.05,9]nonadeca-5(9),6-dien-17-yl]methanol | ||
| SMILES | CC12CCC3=C(C1CCC45C2CCC(C4)C(C5)(CO)OC)C=CO3 | ||
| Standard InChIKey | BDVVNPOGDNWUOI-PUYAIYPXSA-N | ||
| Standard InChI | InChI=1S/C21H30O3/c1-19-8-6-17-15(7-10-24-17)16(19)5-9-20-11-14(3-4-18(19)20)21(12-20,13-22)23-2/h7,10,14,16,18,22H,3-6,8-9,11-13H2,1-2H3/t14?,16?,18?,19-,20+,21+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

16-O-Methylcafestol Dilution Calculator

16-O-Methylcafestol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0257 mL | 15.1286 mL | 30.2572 mL | 60.5144 mL | 75.643 mL |
| 5 mM | 0.6051 mL | 3.0257 mL | 6.0514 mL | 12.1029 mL | 15.1286 mL |
| 10 mM | 0.3026 mL | 1.5129 mL | 3.0257 mL | 6.0514 mL | 7.5643 mL |
| 50 mM | 0.0605 mL | 0.3026 mL | 0.6051 mL | 1.2103 mL | 1.5129 mL |
| 100 mM | 0.0303 mL | 0.1513 mL | 0.3026 mL | 0.6051 mL | 0.7564 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 6-Methoxytricin
Catalog No.:BCN0334
CAS No.:76015-42-4
- (+)-Lupanine hydrochloride
Catalog No.:BCN0333
CAS No.:1025-39-4
- Lavandulyl acetate
Catalog No.:BCN0332
CAS No.:25905-14-0
- Lavandulol
Catalog No.:BCN0331
CAS No.:58461-27-1
- Lactucin
Catalog No.:BCN0330
CAS No.:1891-29-8
- Isoxanthohumol
Catalog No.:BCN0329
CAS No.:521-48-2
- Isomenthone
Catalog No.:BCN0328
CAS No.:491-07-6
- Hyperforin (stable Dicyclohexylammonium salt)
Catalog No.:BCN0327
CAS No.:238074-03-8
- 3'-Hydroxyflavone
Catalog No.:BCN0326
CAS No.:70460-18-3
- (Rac)-Hesperetin
Catalog No.:BCN0325
CAS No.:69097-99-0
- Grindelic acid
Catalog No.:BCN0324
CAS No.:1438-57-9
- Fukinolic acid
Catalog No.:BCN0323
CAS No.:50982-40-6
- Naringenin chalcone
Catalog No.:BCN0336
CAS No.:25515-46-2
- Pinocembroside
Catalog No.:BCN0337
CAS No.:75829-43-5
- (S)-4',5,7-Trihydroxy-6-prenylflavanone
Catalog No.:BCN0338
CAS No.:68682-01-9
- Sophoraflavanone B
Catalog No.:BCN0339
CAS No.:68682-02-0
- Rebaudioside I
Catalog No.:BCN0340
CAS No.:1220616-34-1
- Rebaudioside O
Catalog No.:BCN0341
CAS No.:1220616-48-7
- Sabinene
Catalog No.:BCN0342
CAS No.:3387-41-5
- (RS)-Sakuranetin
Catalog No.:BCN0343
CAS No.:520-29-6
- trans-Sinapic acid
Catalog No.:BCN0344
CAS No.:7362-37-0
- Solanthrene
Catalog No.:BCN0345
CAS No.:26516-51-8
- Cannabisin G
Catalog No.:BCN0346
CAS No.:
- Tabersonine hydrochloride
Catalog No.:BCN0347
CAS No.:29479-00-3
Quantitative NMR Methodology for the Authentication of Roasted Coffee and Prediction of Blends.[Pubmed:33252222]
J Agric Food Chem. 2020 Dec 9;68(49):14643-14651.
In response to the need from the food industry for new analytical solutions, a fit-for-purpose quantitative (1)H NMR methodology was developed to authenticate pure coffee (100% arabica or robusta) as well as predict the percentage of robusta in blends through the study of 292 roasted coffee samples in triplicate. Methanol was chosen as the extraction solvent, which led to the quantitation of 12 coffee constituents: caffeine, trigonelline, 3- and 5-caffeoylquinic acid, lipids, cafestol, nicotinic acid, N-methylpyridinium, formic acid, acetic acid, kahweol, and 16-O-Methylcafestol. To overcome the chemical complexity of the methanolic extract, quantitative analysis was performed using a combination of traditional integration and spectral deconvolution methods. As a result, the proposed methodology provides a systematic methodology and a linear regression model to support the classification of known and unknown roasted coffees and their blends.
16-O-Methylated diterpenes in green Coffea arabica: ultra-high-performance liquid chromatography-tandem mass spectrometry method optimization and validation.[Pubmed:32767433]
J Mass Spectrom. 2020 Nov;55(11):e4636.
Coffee diterpenes are the main constituents of the coffee oil unsaponifiable fraction. The three most important diterpenes are cafestol, kahweol, and 16-O-Methylcafestol (16-OMC), and they are produced, except for cafestol, only by plants of the Coffea genus. Recently, in addition to these three major diterpenes, another 16-O-methylated diterpene (16-O-methylkahweol: 16-OMK) has been identified and quantified, for the first time, in Robusta coffee. For many years, 16-OMC has been considered present exclusively in Robusta, and so it has been reputed an excellent authenticity marker for the presence of Robusta in coffee products. For its quantification, nuclear magnetic resonance (NMR) has proved very useful when compared with other methods. Quite recently, the detection of very low levels of the two 16-O-methylated diterpenes (16-OMD) 16-OMC and 16-OMK in roasted Arabica was reported. This finding makes the use of NMR methods in 16-OMD quantification in Arabica coffee particularly challenging in view of both the trace amounts of 16-OMD and the impossibility to discriminate between 16-OMC and 16-OMK. The ultra-high performance liquid chromatography mass spectrometry (UHPLC-MS) method, already used to detect 16-OMC and 16-OMK in Arabica roasted coffee, is then more suitable for quantitative analyses. Up to now however, no quantification of coffee 16-OMD via ultra-high performance liquid chromatography tandem mass spectrometry (UHPLC-MS/MS) has been carried out; this largely stimulated the present study. For the first time, a simple procedure for the quantitative detection of 16-OMD in Arabica coffee has been developed, and as far as 16-OMC is concerned, fully validated in terms of specificity, linearity, concentration range, limit of detection (LOD), limit of quantification (LOQ), and repeatability following the criteria specified in the EU Commission Decision 2002/675/EC. This method proved to be very specific and sensitive. In order to avoid the chemical complexity generated by the roasting process, the method was optimized and validated on several green Arabica samples from different geographical origins.
Limited genotypic and geographic variability of 16-O-methylated diterpene content in Coffea arabica green beans.[Pubmed:32497844]
Food Chem. 2020 Nov 1;329:127129.
The acknowledged marker of Robusta coffee, 16-O-Methylcafestol (16-OMC), can be quantified by NMR as a mixture with 16-O-methylkahweol (16-OMK), which accounts for approximately 10% of the mixture. In the present study, we detected and quantified 16-O-methylated diterpenes (16-OMD) in 248 samples of green Coffea arabica beans by NMR. We did not observe any differences between genotypes introgressed by chromosomal fragments of Robusta and non-introgressed genotypes. Environmental effects suggesting a possible protective role of 16-OMD for adaptation, as well as genotypic effects that support a high heritability of this trait were observed. Altogether, our data confirmed the presence of 16-OMD in green Arabica at a level approximately 1.5% that of a typical Robusta, endorsing the validity of 16-OMD as a marker for the presence of Robusta.
Interaction of the Coffee Diterpenes Cafestol and 16-O-Methyl-Cafestol Palmitates with Serum Albumins.[Pubmed:32155814]
Int J Mol Sci. 2020 Mar 6;21(5). pii: ijms21051823.
The main coffee diterpenes cafestol, kahweol, and 16-O-Methylcafestol, present in the bean lipid fraction, are mostly esterified with fatty acids. They are believed to induce dyslipidaemia and hypercholesterolemia when taken with certain types of coffee brews. The study of their binding to serum albumins could help explain their interactions with biologically active xenobiotics. We investigated the interactions occurring between cafestol and 16-O-Methylcafestol palmitates with Bovine Serum Albumin (BSA), Human Serum Albumin (HSA), and Fatty Free Human Serum Albumin (ffHSA) by means of circular dichroism and fluorimetry. Circular Dichroism (CD) revealed a slight change (up to 3%) in the secondary structure of fatty-free human albumin in the presence of the diterpene esters, suggesting that the aliphatic chain of the palmitate partly occupies one of the fatty acid sites of the protein. A warfarin displacement experiment was performed to identify the binding site, which is probably close but not coincident with Sudlow site I, as the affinity for warfarin is enhanced. Fluorescence quenching titrations revealed a complex behaviour, with Stern-Volmer constants in the order of 10(3)-10(4) Lmol(-1). A model of the HSA-warfarin-cafestol palmitate complex was obtained by docking, and the most favourable solution was found with the terpene palmitate chain inside the FA4 fatty acid site and the cafestol moiety fronting warfarin at the interface with site I.
Validation of a Quantitative Proton Nuclear Magnetic Resonance Spectroscopic Screening Method for Coffee Quality and Authenticity (NMR Coffee Screener).[Pubmed:31947906]
Foods. 2020 Jan 4;9(1). pii: foods9010047.
Monitoring coffee quality as a means of detecting and preventing economically motivated fraud is an important aspect of international commerce today. Therefore, there is a compelling need for rapid high throughput validated analytical techniques such as quantitative proton nuclear magnetic resonance (NMR) spectroscopy for screening and authenticity testing. For this reason, we sought to validate an (1)H NMR spectroscopic method for the routine screening of coffee for quality and authenticity. A factorial experimental design was used to investigate the influence of the NMR device, extraction time, and nature of coffee on the content of caffeine, 16-O-Methylcafestol (OMC), kahweol, furfuryl alcohol, and 5-hydroxymethylfurfural (HMF) in coffee. The method was successfully validated for specificity, selectivity, sensitivity, and linearity of detector response. The proposed method produced satisfactory precision for all analytes in roasted coffee, except for kahweol in canephora (robusta) coffee. The proposed validated method may be used for routine screening of roasted coffee for quality and authenticity control (i.e., arabica/robusta discrimination), as its applicability was demonstrated during the recent OPSON VIII Europol-Interpol operation on coffee fraud control.
16-O-methylcafestol is present in ground roast Arabica coffees: Implications for authenticity testing.[Pubmed:29329870]
Food Chem. 2018 May 15;248:52-60.
High-field and low-field proton NMR spectroscopy were used to analyse lipophilic extracts from ground roast coffees. Using a sample preparation method that produced concentrated extracts, a small marker peak at 3.16ppm was observed in 30 Arabica coffees of assured origin. This signal has previously been believed absent from Arabicas, and has been used as a marker for detecting adulteration with robusta. Via 2D 600MHz NMR and LC-MS, 16-O-Methylcafestol and 16-O-methylkahweol were detected for the first time in Arabica roast coffee and shown to be responsible for the marker peak. Using low-field NMR, robusta in Arabica could be detected at levels of the order of 1-2%w/w. A surveillance study of retail purchased "100% Arabica" coffees found that 6 out of 60 samples displayed the 3.16ppm marker signal to a degree commensurate with adulteration at levels of 3-30%w/w.
Screening for 16-O-methylcafestol in roasted coffee by high-performance thin-layer chromatography-fluorescence detection - Determination of Coffea canephora admixtures to Coffea arabica.[Pubmed:29042111]
J Chromatogr A. 2017 Nov 24;1525:173-180.
16-O-Methylcafestol (16-OMC), the characteristic diterpene exclusively present in Coffea canephora, is an excellent marker for Coffea canephora admixtures to Coffea arabica. Here we show a straightforward, selective and sensitive screening method for the determination of 16-OMC in roasted coffee by high-performance thin-layer chromatography with fluorescence detection (HPTLC-FLD). As internal standard, Sudan IV was used, and a direct saponification with 10% ethanolic potassium hydroxide solution was followed by solid supported liquid extraction with petroleum ether. 16-OMC was selectively derivatized with 2-naphthoyl chloride and analyzed by HPTLC-FLD on silica gel plates with cyclohexane/tert-butyl methyl ether/formic acid (86:14:2, v/v/v) as the mobile phase. The enhanced fluorescence was scanned at UV 244/>320nm. Limits of detection and quantitation of 5 and 14mg 16-OMC/kg coffee allowed the determination of Coffea canephora admixtures to Coffea arabica below 1%. Recoveries for blends of Coffea arabica with Coffea canephora were close to 100%.
Low-field (1)H NMR spectroscopy for distinguishing between arabica and robusta ground roast coffees.[Pubmed:27596398]
Food Chem. 2017 Feb 1;216:106-13.
This work reports a new screening protocol for addressing issues of coffee authenticity using low-field (60MHz) bench-top (1)H NMR spectroscopy. Using a simple chloroform-based extraction, useful spectra were obtained from the lipophilic fraction of ground roast coffees. It was found that 16-O-Methylcafestol (16-OMC, a recognized marker compound for robusta beans) gives rise to an isolated peak in the 60MHz spectrum, which can be used as an indicator of the presence of robusta beans in the sample. A total of 81 extracts from authenticated coffees and mixtures were analysed, from which the detection limit of robusta in arabica was estimated to be between 10% and 20% w/w. Using the established protocol, a surveillance exercise was conducted of 27 retail samples of ground roast coffees which were labelled as "100% arabica". None were found to contain undeclared robusta content above the estimated detection limit.
Interaction of coffee compounds with serum albumins. Part II: Diterpenes.[Pubmed:26776001]
Food Chem. 2016 May 15;199:502-8.
Cafestol and 16-O-Methylcafestol are diterpenes present in coffee, but whilst cafestol is found in both Coffea canephora and Coffea arabica, 16-O-Methylcafestol (16-OMC) was reported to be specific of only C. canephora. The interactions of such compounds, with serum albumins, have been studied. Three albumins have been considered, namely human serum albumin (HSA), fatty acid free HSA (ffHSA) and bovine serum albumin (BSA). The proteins interact with the diterpenes at the interface between Sudlow site I and the fatty acid binding site 6 in a very peculiar way, leading to a significant change in the secondary structure. The diterpenes do not displace reference binding drugs of site 2, but rather they enhance the affinity of the site for the drugs. They, therefore, may alter the pharmacokinetic profile of albumin - bound drugs.
Rapid approach to identify the presence of Arabica and Robusta species in coffee using 1H NMR spectroscopy.[Pubmed:25842325]
Food Chem. 2015 Sep 1;182:178-84.
NMR spectroscopy was used to verify the presence of Arabica and Robusta species in coffee. Lipophilic extracts of authentic roasted and green coffees showed the presence of established markers for Robusta (16-O-Methylcafestol (16-OMC)) and for Arabica (kahweol). The integration of the 16-OMC signal (delta 3.165 ppm) was used to estimate the amount of Robusta in coffee blends with an approximate limit of detection of 1-3%. The method was successfully applied for the analysis of 77 commercial coffee samples (coffee pods, coffee capsules, and coffee beans). Furthermore, principal component analysis (PCA) was applied to the spectra of lipophilic and aqueous extracts of 20 monovarietal authentic samples. Clusters of the two species were observed. NMR spectroscopy can be used as a rapid prescreening tool to discriminate Arabica and Robusta coffee species before the confirmation applying the official method.
Rapid authentication of coffee blends and quantification of 16-O-methylcafestol in roasted coffee beans by nuclear magnetic resonance.[Pubmed:25431971]
J Agric Food Chem. 2014 Dec 24;62(51):12309-14.
Roasted coffee is subject to commercial frauds, because the high-quality Coffea arabica species, described as "100% Arabica" or "Highland coffee", is often mixed with the less expensive Coffea canephora var. Robusta. The quantification of 16-O-Methylcafestol (16-OMC) is useful to monitor the authenticity of the products as well as the Robusta content in blends. The German standard method DIN 10779 is used in the determination of 16-OMC in roasted coffee beans to detect C. canephora in blends, but it is laborious and time-consuming. Here, we introduce a new method that provides a quantitative determination of esterified 16-OMC directly in coffee extracts by means of high-resolution proton nuclear magnetic resonance spectroscopy. Limit of detection and limit of quantitation were 5 and 20 mg/kg, respectively, which are adequate to detect the presence of Robusta at percentages lower than 0.9%. The proposed method is much faster, more sensitive, and much more reproducible than the DIN standard method.
Green coffee oil analysis by high-resolution nuclear magnetic resonance spectroscopy.[Pubmed:23618184]
Talanta. 2013 Jun 15;110:118-27.
In this work, we show how an extensive and fast quantification of the main components in green coffee oil can be achieved by NMR, with minimal sample manipulation and use of organic solvents. The approach is based on the integration of characteristic NMR signals, selected because of their similar relaxation properties and because they fall in similar spectral regions, which minimizes offset effects. Quantification of glycerides, together with their fatty acid components (oleic, linoleic, linolenic and saturated) and minor species (caffeine, cafestol, kahweol and 16-O-Methylcafestol), is achieved in less than 1h making use of (1)H and (13)C spectroscopy. The compositional data obtained are in reasonable agreement with classical chromatographic analyses.
Authentication of Italian Espresso coffee blends through the GC peak ratio between kahweol and 16-O-methylcafestol.[Pubmed:22953895]
Food Chem. 2012 Dec 1;135(3):1569-74.
Since the price of Arabica is currently more than twice higher than Robusta, a rapid and reliable method for the determination of the roasted coffee blend composition is fundamental for the authentication of commercial blends used for the Italian Espresso coffee. A GC-FID method based on the ratio between the integrated peak areas of kahweol (K) divided by the sum of K and 16-O-Methylcafestol (16MCF) was developed. No internal/external standard was used. Moreover, the quantitation of the unsaponifiable compounds is not necessary, as well as the calculation of any response factors. The percentage of Robusta in 34 samples of coffee blends with known composition, and in 48 samples of pure varieties was used to build a cubic polynomial function with R(2)=0.998. The roasting conditions did not affect the results. Considering eight commercial blends (ranging 0-90% Robusta), no significant difference (two-tailed P=0.817) was registered between the claimed and the predicted composition.


