1-DeoxyforskolinCAS# 72963-77-0 |
Quality Control & MSDS
Number of papers citing our products

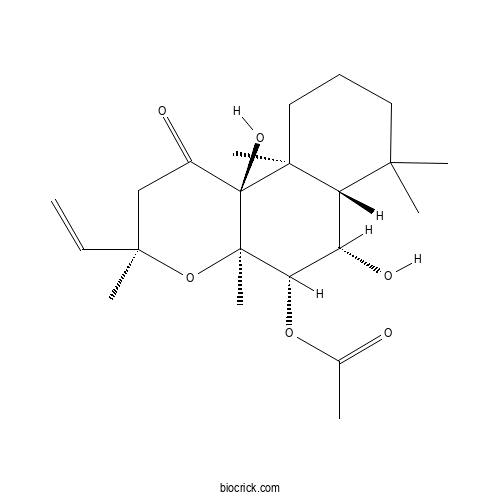
Chemical structure

3D structure
| Cas No. | 72963-77-0 | SDF | Download SDF |
| PubChem ID | 14219439.0 | Appearance | Powder |
| Formula | C22H34O6 | M.Wt | 394.5 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | [(3R,4aR,5S,6S,6aS,10aS,10bS)-3-ethenyl-6,10b-dihydroxy-3,4a,7,7,10a-pentamethyl-1-oxo-5,6,6a,8,9,10-hexahydro-2H-benzo[f]chromen-5-yl] acetate | ||
| SMILES | CC(=O)OC1C(C2C(CCCC2(C3(C1(OC(CC3=O)(C)C=C)C)O)C)(C)C)O | ||
| Standard InChIKey | PXYAFNGUEZPJBI-OAUGPMCWSA-N | ||
| Standard InChI | InChI=1S/C22H34O6/c1-8-19(5)12-14(24)22(26)20(6)11-9-10-18(3,4)16(20)15(25)17(27-13(2)23)21(22,7)28-19/h8,15-17,25-26H,1,9-12H2,2-7H3/t15-,16-,17-,19-,20-,21+,22-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

1-Deoxyforskolin Dilution Calculator

1-Deoxyforskolin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5349 mL | 12.6743 mL | 25.3485 mL | 50.6971 mL | 63.3714 mL |
| 5 mM | 0.507 mL | 2.5349 mL | 5.0697 mL | 10.1394 mL | 12.6743 mL |
| 10 mM | 0.2535 mL | 1.2674 mL | 2.5349 mL | 5.0697 mL | 6.3371 mL |
| 50 mM | 0.0507 mL | 0.2535 mL | 0.507 mL | 1.0139 mL | 1.2674 mL |
| 100 mM | 0.0253 mL | 0.1267 mL | 0.2535 mL | 0.507 mL | 0.6337 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 2,3-Dimethoxybenzoic acid
Catalog No.:BCX0622
CAS No.:1521-38-6
- 6-Deoxy-D-glucose
Catalog No.:BCX0621
CAS No.:7658-08-4
- trans-4-Methoxycinnamic acid
Catalog No.:BCX0620
CAS No.:943-89-5
- Saikogenin F
Catalog No.:BCX0619
CAS No.:14356-59-3
- 11-Oxo-Alpha-Amyrin
Catalog No.:BCX0618
CAS No.:2118-90-3
- 2-Methoxycinnamaldehyde
Catalog No.:BCX0617
CAS No.:60125-24-8
- 3’,5-dihydroxy-2-(4-hydroxybenzyl)3-methoxybibenzyl
Catalog No.:BCX0616
CAS No.:151538-57-7
- 3,4,5-Trimethoxytoluene
Catalog No.:BCX0615
CAS No.:6443-69-2
- Resveratrol-4'-O-β-D-(6''-O-galloy)-glucopyranoside
Catalog No.:BCX0614
CAS No.:64898-03-9
- 3,5-Dimethoxytoluene
Catalog No.:BCX0613
CAS No.:4179-19-5
- Oleanic aldehyde
Catalog No.:BCX0612
CAS No.:17020-22-3
- OJV-VI
Catalog No.:BCX0611
CAS No.:125150-67-6
- 4-Methoxy-1,2-benzenediol
Catalog No.:BCX0624
CAS No.:3934-97-2
- Yibeinoside A
Catalog No.:BCX0625
CAS No.:98985-24-1
- γ-sanshool
Catalog No.:BCX0626
CAS No.:78886-65-4
- (+)-Balanophonin
Catalog No.:BCX0627
CAS No.:215319-47-4
- Blestriarene A
Catalog No.:BCX0628
CAS No.:126721-53-7
- Creatinine
Catalog No.:BCX0629
CAS No.:60-27-5
- 1,1,1,1-Kestohexose
Catalog No.:BCX0630
CAS No.:62512-19-0
- Uric acid
Catalog No.:BCX0631
CAS No.:69-93-2
- Monoethyl fumaric acid
Catalog No.:BCX0632
CAS No.:2459-05-4
- N-Methyl-1-deoxynojirimycin
Catalog No.:BCX0633
CAS No.:69567-10-8
- Ocimene
Catalog No.:BCX0634
CAS No.:13877-91-3
- Manzamine A
Catalog No.:BCX0635
CAS No.:104196-68-1
Impact of seasonal variation on four labdane-type diterpenoids in Coleus forskholii Briq.[Pubmed:36695524]
Nat Prod Res. 2023 Jan 25:1-6.
The present study has been planned to evaluate the impact of seasonal variation in labdane-type diterpenoids namely isoforskolin, forskolin, 1,9-dideoxyforskolin and 1-Deoxyforskolin in Coleus forskholii (roots). The plant samples were harvested in different seasons from our experimental field located at CSIR-NBRI garden, Lucknow (India) and metabolite contents were estimated through validated high performance thin layer chromatography (HPTLC) method. The HPTLC plate was developed in tertiary mobile phase of toluene-ethyl acetate-methanol (8.5-1-0.05 v/v) for separation of all the four metabolites. The metabolite content viz. isoforskolin, forskolin, 1,9-dideoxyforskolin and 1-Deoxyforskolin varies from 0.0247% to 0.198%, 0.238 to 0.730%, 0.056 to 0.161% and 0.0401 to 0.332% on dry weight basis respectively. The maximum content of metabolites was recorded in winter season and was found optimum for harvesting of C. forskholii roots. Optimization of harvesting season for this industrially valuable medicinal plant will lead to sustainable sources of good quality raw material to herbal drug industry.
Effect of Coleus forskohlii and its major constituents on cytochrome P450 induction.[Pubmed:26870691]
J Tradit Complement Med. 2015 Jan 29;6(1):130-3.
Coleus forskohlii Briq. has been used traditionally for the treatment of several ailments since antiquity in Ayurveda. In the present study, an approach has been made to evaluate the effect of C. forskohlii and its major constituents on cytochrome P450 (CYP3A, CYP2B, and CYP2C) mRNA expression in rat hepatocytes. To gain better understanding of the herb-drug interaction potential of the chemical constituents present in C. forskohlii, the extract was subjected to column chromatography followed by standardization with respect to forskolin, 1-Deoxyforskolin, and 1,9-dideoxyforskolin using reversed-phase high-performance liquid chromatography (RP-HPLC). Hepatocytes were treated with extracts, fractions, and phytoconstituents, followed by extraction and purification of total mRNA. Study of mRNA expression was carried out through reverse transcription polymerase chain reaction, followed by agarose gel electrophoresis. Results revealed that the test substances did not show any significant mRNA expression compared to the control against CYP3A, CYP2B, and CYP2C. Positive controls such as dexamethasone and rifampin showed significantly high (p < 0.001) induction potential compared to the control. It can be concluded that C. forskohlii and its major constituents may not be involved in CYP450 induction-based drug interaction.
[Studies on the chemical constituents of Coleus forskohlii].[Pubmed:20034210]
Zhong Yao Cai. 2009 Sep;32(9):1381-5.
OBJECTIVE: To study the chemical constituents in the aerial parts of Coleus forskohlii. METHODS: The compounds were isolated by various column chromatographic methods, and their structures were identified by spectroscopic methods. RESULTS: Twelve compounds were isolated and identified as chamaecydin (1), 6 alpha-hydroxydemethylcryptojaponol (2), alpha-cedrene (3), oleanolic acid (4), forskolin G (5), forskolin J (6), 1,6-diacetyl-9-deoxyforskolin (7), forskolin A (8), forskolin H (9), 6-acetyl-1-Deoxyforskolin (10), betulinic acid (11), beta-sitosterol (12). CONCLUSION: Compounds 1 - 3 are isolated from Coleus genus for the first time, and compound 4 is isolated from C. forskohlii for the first time.
Anti-HIV diterpenes from Coleus forskohlii.[Pubmed:19831022]
Nat Prod Commun. 2009 Sep;4(9):1173-5.
Various extracts of the aerial parts of Coleus forskohlii (Labiatae) were prepared and evaluated at their non cytotoxic concentration against HIV-1 NL4-3. Chloroform, ethyl acetate and n-butanol extracts showed 45.6, 66.5 and 37.7% inhibition of HIV, respectively in CEM-GFP cells infected with HIV-1(NL4-3) at 5 microg/mL. Four diterpenes, 1-Deoxyforskolin, 1,9-dideoxyforskolin, forskolin and isoforskolin were isolated from the chloroform extract and tested against the virus. Six semi-synthetic derivatives of forskolin have been prepared to study SAR. 1-Deoxyforskolin and forskolin were found to be active against HIV(NL4-3). This is first report of anti HIV activity of this plant and its isolated constituents.
Carbon-11-forskolin: a ligand for visualization of the adenylate cyclase-related second messenger system.[Pubmed:8229239]
J Nucl Med. 1993 Nov;34(11):1944-8.
To visualize the adenylate cyclase (AC)-related second messenger system, [11C]forskolin, [11C]1-acetyl-7-deacetylforskolin, [11C]1,9-dideoxyforskolin and [11C]1-Deoxyforskolin were synthesized by acetylation of the respective deacetyl-precursors using [11C]acetylchloride and dimethylaminopyridine. The radiochemical yield of [11C]forskolin, [11C]1-acetyl-7-deacetylforskolin, [11C]1,9-dideoxyforskolin and [11C]1-Deoxyforskolin calculated from trapped [11C]CO2 were 5%, 10%, 15% and 18%, respectively. Since the 1- and 9-OH groups on the forskolin structure are critical for specific binding to AC (active type), we considered [11C]1-acetyl-7-deacetylforskolin, [11C]1,9-dideoxyforskolin and [11C]1-Deoxyforskolin to be nonspecific forskolin analogs. A comparative study of [11C]forskolin and its analogs on the n-octanol/phosphate buffer (pH 7.4) partition ratio showed that [11C]1-acetyl-7-deacetylforskolin has similar physical properties to [11C]forskolin. In the mouse heart, kidneys, liver and lungs, more [11C]forskolin accumulated than [11C]1-acetyl-7-deacetylforskolin. Moreover, simultaneous [11C]forskolin with forskolin (10 micrograms) administration reduced the accumulation of [11C]forskolin particularly in the heart to the level of [11C]1-acetyl-7-deacetylforskolin. These results indicate that [11C]forskolin would be a useful imaging agent for the AC-related second messenger system.
Selective modulation of vinblastine sensitivity by 1,9-dideoxyforskolin and related diterpenes in multidrug resistant tumour cells.[Pubmed:8094975]
Br J Cancer. 1993 Mar;67(3):471-9.
The ability of 1,9-dideoxyforskolin (DDF), 1-Deoxyforskolin (DF) and forskolin to modulate cellular sensitivity to vinblastine (VBL) was examined in drug-sensitive parental KB-3-1 cells and a multidrug-resistant subline, KB-GRC1, derived by transfection of mdr1. Fifty microM DF and forskolin enhanced the 1 h uptake of VBL by 8.0 +/- 0.7 (s.d.) and 4.7 +/- 2.5-fold, respectively, with 50 microM DDF producing a 13.6 +/- 1.9-fold increase. The greater effect of DDF relative to forskolin indicated that the effect was independent of activation of cAMP, and this was supported by a lack of effect of dibutyryl cAMP on the uptake. The effect of these agents on uptake were < or = 1.4-fold in KB-3-1 cells. DDF selectively inhibited initial efflux in cells expressing a functional P-glycoprotein (PGP), but both forskolin and DDF inhibited the terminal phase of efflux irrespective of PGP expression. Neither agent affected membrane permeability of polarisation and forskolin did not enhance the uptake of VBL in protein-free liposomes. At a non-toxic concentration of 20 microM, DDF and forskolin decreased the IC50 of VBL from 18.9 to 2.7 and 13 nM in KB-GRC1 cells, respectively, and DDF acted synergistically with VBL as shown by median effect analysis [combination index = 0.20 +/- 0.05 (s.d.)]. In contrast, these diterpenes did not affect VBL sensitivity in KB-3-1 cells. These results indicate that the diterpenes modulate VBL sensitivity predominantly by inhibiting PGP-mediated efflux activity.


