1-Caffeoylquinic acidCAS# 1241-87-8 |
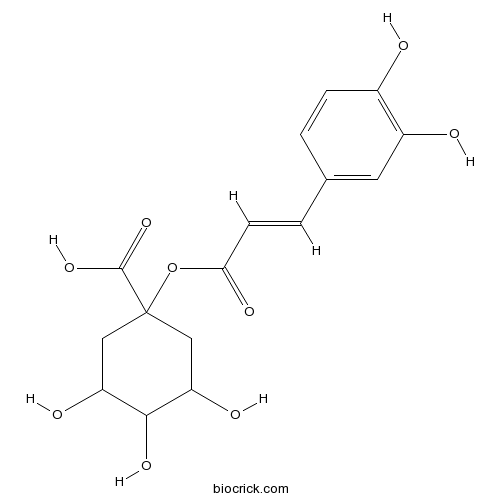
- cis-1-o-Caffeoylquinic acid
Catalog No.:BCN6390
CAS No.:1627537-95-4
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 1241-87-8 | SDF | Download SDF |
| PubChem ID | 6451212 | Appearance | White powder |
| Formula | C16H18O9 | M.Wt | 354.31 |
| Type of Compound | Phenylpropanoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 1-[(E)-3-(3,4-dihydroxyphenyl)prop-2-enoyl]oxy-3,4,5-trihydroxycyclohexane-1-carboxylic acid | ||
| SMILES | C1C(C(C(CC1(C(=O)O)OC(=O)C=CC2=CC(=C(C=C2)O)O)O)O)O | ||
| Standard InChIKey | GWTUHAXUUFROTF-DUXPYHPUSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1-Caffeoylquinic acid is an important intermediate in lignin biosynthesis. 1-Caffeoylquinic acid has anti-influenza, and antioxidant activities, it also slows the release of glucose into the bloodstream after a meal. |
| Targets | NF-kB |
| In vitro | Qualitative and quantitative analysis of phenolics in Tetrastigma hemsleyanum and their antioxidant and antiproliferative activities.[Pubmed: 24151872]J Agric Food Chem. 2013 Nov 6;61(44):10507-15.The phenolic profiles of Tetrastigma hemsleyanum leaf extracts by different solvents (80% methanol, ethyl acetate and hexane) and their antioxidant and antiproliferative activities were investigated. |
| Kinase Assay | Dietary phytochemicals as potent chemotherapeutic agents against breast cancer: Inhibition of NF-κB pathway via molecular interactions in rel homology domain of its precursor protein p105.[Pubmed: 23661994]Pharmacogn Mag. 2013 Jan;9(33):51-7.Dietary phytochemicals consist of a wide variety of biologically active compounds that are ubiquitous in plants, many of which have been reported to have anti-tumor as well as anti-inflammatory properties.
In the present study, we aimed to validate these findings by using docking protocols and explicate the possible mechanism of action for a dataset of nine phytochemicals namely boswellic acid, 1-Caffeoylquinic acid, ellagic acid, emodin, genistein, guggulsterone, quercetin, resveratrol, and sylibinin from different plants against the nuclear factor- kappaB (NF-κB) precursor protein p105, an important transcription factor reported to be overexpressed in breast cancer.
|

1-Caffeoylquinic acid Dilution Calculator

1-Caffeoylquinic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8224 mL | 14.1119 mL | 28.2239 mL | 56.4477 mL | 70.5597 mL |
| 5 mM | 0.5645 mL | 2.8224 mL | 5.6448 mL | 11.2895 mL | 14.1119 mL |
| 10 mM | 0.2822 mL | 1.4112 mL | 2.8224 mL | 5.6448 mL | 7.056 mL |
| 50 mM | 0.0564 mL | 0.2822 mL | 0.5645 mL | 1.129 mL | 1.4112 mL |
| 100 mM | 0.0282 mL | 0.1411 mL | 0.2822 mL | 0.5645 mL | 0.7056 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- (-)-Hydroxydihydrobovolide
Catalog No.:BCN7890
CAS No.:124097-54-7
- 16-Epikoumidine
Catalog No.:BCN3915
CAS No.:124096-81-7
- Etomoxir
Catalog No.:BCC1564
CAS No.:124083-20-1
- 7',8'-Dihydroobolactone
Catalog No.:BCN7196
CAS No.:1240403-82-0
- AZD3514
Catalog No.:BCC1070
CAS No.:1240299-33-5
- Kobophenol A
Catalog No.:BCN3444
CAS No.:124027-58-3
- 1beta,10beta-Epoxydesacetoxymatricarin
Catalog No.:BCN7307
CAS No.:124020-39-9
- Triamcinolone
Catalog No.:BCC4741
CAS No.:124-94-7
- Oxycodone hydrochloride
Catalog No.:BCC6090
CAS No.:124-90-3
- Picrotoxin
Catalog No.:BCC5705
CAS No.:124-87-8
- Isoborneol
Catalog No.:BCN7158
CAS No.:124-76-5
- Decane
Catalog No.:BCN8138
CAS No.:124-18-5
- 2-Hydroxytetracosanoic acid ethyl ester
Catalog No.:BCN1599
CAS No.:124111-47-3
- Scutebarbatine O
Catalog No.:BCN8377
CAS No.:960302-88-9
- Alcesefoliside
Catalog No.:BCN2933
CAS No.:124151-38-8
- (R)-DRF053 dihydrochloride
Catalog No.:BCC7726
CAS No.:1241675-76-2
- 6-O-Vanilloylajugol
Catalog No.:BCN6125
CAS No.:124168-04-3
- Laxiracemosin H
Catalog No.:BCN6910
CAS No.:1241871-28-2
- 12-Ursene-3,16,22-triol
Catalog No.:BCN6126
CAS No.:1242085-06-8
- RN486
Catalog No.:BCC3921
CAS No.:1242156-23-5
- Wnt-C59
Catalog No.:BCC3965
CAS No.:1243243-89-1
- LGK-974
Catalog No.:BCC5103
CAS No.:1243244-14-5
- Paucinervin A
Catalog No.:BCN7308
CAS No.:1243249-16-2
- Lenalidomide hydrochloride
Catalog No.:BCC1697
CAS No.:1243329-97-6
Dietary phytochemicals as potent chemotherapeutic agents against breast cancer: Inhibition of NF-kappaB pathway via molecular interactions in rel homology domain of its precursor protein p105.[Pubmed:23661994]
Pharmacogn Mag. 2013 Jan;9(33):51-7.
BACKGROUND: Dietary phytochemicals consist of a wide variety of biologically active compounds that are ubiquitous in plants, many of which have been reported to have anti-tumor as well as anti-inflammatory properties. OBJECTIVE: In the present study, we aimed to validate these findings by using docking protocols and explicate the possible mechanism of action for a dataset of nine phytochemicals namely boswellic acid, 1-Caffeoylquinic acid, ellagic acid, emodin, genistein, guggulsterone, quercetin, resveratrol, and sylibinin from different plants against the nuclear factor- kappaB (NF-kappaB) precursor protein p105, an important transcription factor reported to be overexpressed in breast cancer. MATERIALS AND METHODS: 2-D structures of all phytochemicals were retrieved from PubChem Compound database and their subsequent conversion into 3-D structures was performed by using online software system CORINA. The X-ray crystallographic structure of the NF-kappaB precursor p105 was extracted from Brookhaven Protein Data Bank. Molecular docking simulation study was carried out by using AutoDock Tools 4.0. RESULTS: Our results showed significant binding affinity of different phytochemicals with the Rel homology domain of the NF-kappaB precursor protein p105. Quercetin and 1-Caffeoylquinic acid were found to be very effective inhibitors against target molecule as they showed binding energy of -12.11 and -11.50 Kcal/mol, respectively. The order of affinity of other ligands with p105 was found as follows: guggulsterone > sylibinin > emodin > resveratrol > genistein > boswellic acid > ellagic acid. CONCLUSION: Our in silico study has explored the possible chemopreventive mechanism of these phytochemicals against the NF-kappaB precursor protein p105 and deciphered that quercetin, 1-Caffeoylquinic acid and guggulsterone were the potent inhibitors against target molecule. In addition, large scale preclinical and clinical trials are needed to explore the role of these chemotherapeutic molecules against the NF-kappaB precursor protein p105 in cure and prevention of breast cancer.
Qualitative and quantitative analysis of phenolics in Tetrastigma hemsleyanum and their antioxidant and antiproliferative activities.[Pubmed:24151872]
J Agric Food Chem. 2013 Nov 6;61(44):10507-15.
The phenolic profiles of Tetrastigma hemsleyanum leaf extracts by different solvents (80% methanol, ethyl acetate and hexane) and their antioxidant and antiproliferative activities were investigated. Thirteen phenolic compounds (3-caffeoylquinic acid, 5-caffeoylquinic acid, 1-Caffeoylquinic acid, 5-p-coumaroylquinic acid, isoorientin-2''-O-rhamnoside, isoorientin, orientin-2''-O-rhamnoside, orientin, 1-p-coumaroylquinic acid, vitexin-2''-O-rhamnoside, isovitexin-2''-O-rhamnoside, vitexin and isovitexin) were identified in T. hemsleyanum leaves for the first time, and six of them were quantified using a combination of LC-QTOF-MS and LC-QqQ-MS techniques. It was found that 80% methanol extract exhibited the highest antioxidant activities (DPPH, 3.32 mmol of Trolox/g DW; ABTS, 1.38 mmol of Trolox/g DW; FRAP, 1.85 mmol of FeSO4/g DW), while the hexane extract had the lowest (1.23, 0.43 and 0.13, respectively). Total phenolic contents (TPC) of various extracts of T. hemsleyanum leaves ranged from 28.95 to 275.71 mg of GAE/g DW. Also, total antioxidant activities as evaluated by ABTS, FRAP and DPPH assays were correlated well with TPC. In addition, 80% methanol extract provided antiproliferative activity on HepG2 cells (IC50 = 524 mug/mL). This paper provides a complete picture of phenolics in T. hemsleyanum leaves and relates them to their antioxidant and antiproliferative activities.


