1,2,3,4,6-O-PentagalloylglucoseCAS# 14937-32-7 |
Quality Control & MSDS
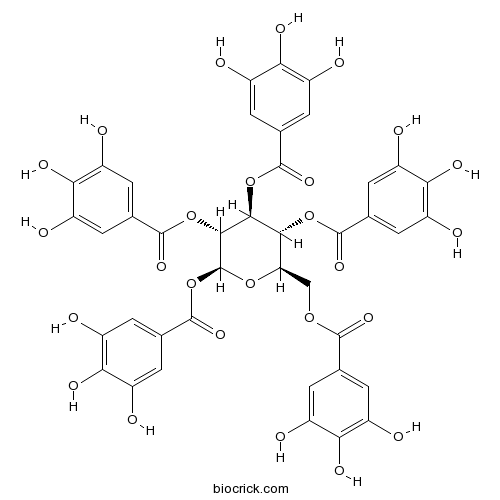
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 14937-32-7 | SDF | Download SDF |
| PubChem ID | 65238 | Appearance | Off-white -beige powder |
| Formula | C41H32O26 | M.Wt | 940.68 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Synonyms | Penta-O-galloyl-β-D-glucose | ||
| Solubility | DMSO : 50 mg/mL (53.15 mM; Need ultrasonic) H2O : 6 mg/mL (6.38 mM; Need ultrasonic and warming) | ||
| Chemical Name | [(2R,3R,4S,5R,6S)-3,4,5,6-tetrakis[(3,4,5-trihydroxybenzoyl)oxy]oxan-2-yl]methyl 3,4,5-trihydroxybenzoate | ||
| SMILES | C1=C(C=C(C(=C1O)O)O)C(=O)OCC2C(C(C(C(O2)OC(=O)C3=CC(=C(C(=C3)O)O)O)OC(=O)C4=CC(=C(C(=C4)O)O)O)OC(=O)C5=CC(=C(C(=C5)O)O)O)OC(=O)C6=CC(=C(C(=C6)O)O)O | ||
| Standard InChIKey | QJYNZEYHSMRWBK-NIKIMHBISA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1,2,3,4,6-O-Pentagalloylglucose(PGG) has antimutagenic, anti-proliferative, anti-invasive,vasodilatory, anti-inflammatory, anti-parasitic, anti-HBV, and antioxidant activities. PGG may serve as a model for the development of new types of anti-diabetic and anti-metabolic syndrome therapeutics. PGG dilates vascular smooth muscle and suppresses the vascular inflammatory process via endothelium-dependent nitric oxide (NO)/cGMP signaling; it also has inhibition of inducible NO synthase and cyclooxygenase-2 activity. |
| Targets | PARP | TNF-α | p65 | NF-kB | COX | NOS | PGE | NO | GLUT | PI3K | Akt | HBV | Antifection |
| In vitro | Study of antimutagenic and antioxidant activities of gallic acid and 1,2,3,4,6-pentagalloylglucose from Pistacia lentiscus. Confirmation by microarray expression profiling.[Pubmed: 17129579 ]Chem.Biol.Interact., 2007, 165(1):1-13.In vitro antioxidant and antimutagenic activities of two polyphenols isolated from the fruits of Pistacia lentiscus was assessed.
Anti-trypanosomal activity of 1,2,3,4,6-penta-O-galloyl-β -D-glucose isolated from Plectranthus barbatus Andrews (Lamiaceae).[Reference: WebLink]Química Nova, 2012, 35(11):2229-332.MeOH extract from the leaves of Plectranthus barbatus Andrews (Lamiaceae), showed in vitro anti-trypanosomal activity.
|
| In vivo | Vasodilatory and anti-inflammatory effects of the 1,2,3,4,6-penta-O-galloyl-beta-D-glucose (PGG) via a nitric oxide-cGMP pathway.[Pubmed: 16253226 ]Eur. J.Pharmacol., 2005, 524(1-3):111-9.Vasorelaxant and anti-inflammatory effects of a 1,2,3,4,6-penta-O-galloyl-beta-d-glucose (1,2,3,4,6-O-Pentagalloylglucose,PGG) isolated from the root barks of Paeonia suffruticosa and possible mechanisms responsible were investigated.
|
| Kinase Assay | Inhibition of inducible nitric oxide synthase and cyclooxygenase-2 activity by 1,2,3,4,6-penta-O-galloyl-beta-D-glucose in murine macrophage cells.[Pubmed: 14609132]Arch Pharm Res. 2003 Oct;26(10):832-9.Activated macrophages express inducible isoforms of nitric oxide synthase (iNOS) and cyclooxygenase (COX-2), and produce excessive amounts of nitric oxide (NO) and prostaglandin E2 (PGE2), which play key roles in the processes of inflammation and carcinogenesis. The root of Paeonia lactiflora Pall., and the root cortex of Paeonia suffruticosa Andr., are important Chinese crude drugs used in many traditional prescriptions. 1,2,3,4,6-penta-O-galloyl-beta-D-glucose (1,2,3,4,6-O-Pentagalloylglucose,PGG) is a major bioactive constituent of both crude drugs. PGG has been shown to possess potent anti-oxidant, anti-mutagenic, anti-proliferative and anti-invasive effects. In this study, we examined the inhibitory effects of 1,2,3,4,6-penta-O-galloyl-beta-D-glucose (PGG) isolated from the root of Paeonia lactiflora Pall. on the COX-2 and iNOS activity in LPS-activated Raw 264.7 cells, COX-1 in HEL cells. To investigate the structure-activity relationships of gallate and gallic acid for the inhibition of iNOS and COX-2 activity, we also examined (-)-epigallocatechin gallate (EGCG), gallic acid, and gallacetophenone. The results of the present study indicated that PGG, EGCG, and gallacetophenone treatment except gallic acid significantly inhibited LPS-induced NO production in LPS-activated macrophages. All of the four compounds significantly inhibited COX-2 activity in LPS-activated macrophages. Among the four compounds examined, PGG revealed the most potent in both iNOS (IC50 approximately 18 microg/mL) and COX-2 inhibitory activity (PGE2: IC50 approximately 8 microg/mL and PGD2: IC50 approximately 12 microg/mL), respectively. Although further studies are needed to elucidate the molecular mechanisms and structure-activity relationship by which PGG exerts its inhibitory actions, our results suggest that PGG might be a candidate for developing anti-inflammatory and cancer chemopreventive agents. |
| Cell Research | In vitro antiviral activity of 1,2,3,4,6-penta-O-galloyl-beta-D-glucose against hepatitis B virus.[Pubmed: 17015965]Natural anti-diabetic compound 1,2,3,4,6-penta-O-galloyl-D-glucopyranose binds to insulin receptor and activates insulin-mediated glucose transport signaling pathway.[Pubmed: 16137651 ]Biochem Biophys Res Commun. 2005 Oct 21;336(2):430-7.Insulin mimetics from natural sources are potential therapeutics that can act alone or supplement insulin and other anti-diabetic drugs in the prevention and treatment of diabetes. We recently reported the insulin-like glucose transport stimulatory activity of tannic acid (TA) in 3T3-L1 adipocytes.
Biol Pharm Bull. 2006 Oct;29(10):2131-4.This study examined the antiviral activity of the root of Paeonia lactiflora PALL.
|

1,2,3,4,6-O-Pentagalloylglucose Dilution Calculator

1,2,3,4,6-O-Pentagalloylglucose Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.0631 mL | 5.3153 mL | 10.6306 mL | 21.2612 mL | 26.5765 mL |
| 5 mM | 0.2126 mL | 1.0631 mL | 2.1261 mL | 4.2522 mL | 5.3153 mL |
| 10 mM | 0.1063 mL | 0.5315 mL | 1.0631 mL | 2.1261 mL | 2.6577 mL |
| 50 mM | 0.0213 mL | 0.1063 mL | 0.2126 mL | 0.4252 mL | 0.5315 mL |
| 100 mM | 0.0106 mL | 0.0532 mL | 0.1063 mL | 0.2126 mL | 0.2658 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
1,2,3,4,6-Penta-O-galloyl-β-D-glucopyranose is a gallotannin isolated from various plants. It suppressed interleukin (IL)-4 induced signal pathway in B cell, and inhibited IgE production partially caused by increasing a population of Treg cells in conjunction with Treg-inducing factors.
References:
[1]. Viswanatha GL, et al. Alleviation of transient global ischemia/reperfusion-induced brain injury in rats with 1,2,3,4,6-penta-O-galloyl-β-d-glucopyranose isolated from Mangifera indica. Eur J Pharmacol. 2013 Nov 15;720(1-3):286-93.
[2]. Kim YH, et al. 1,2,3,4,6-penta-O-galloyl-β-D-glucopyranose increases a population of T regulatory cells and inhibits IgE production in ovalbumin-sensitized mice. Int Immunopharmacol. 2015 May;26(1):30-6.
[3]. Ahn D, et al. The Longevity Properties of 1,2,3,4,6-Penta-O-Galloyl-β-D-Glucose from Curcuma longa in Caenorhabditis elegans. Biomol Ther (Seoul). 2013 Nov;21(6):442-6.
- UNC2250
Catalog No.:BCC4876
CAS No.:1493694-70-4
- AACOCF3
Catalog No.:BCC7075
CAS No.:149301-79-1
- Cytochalasin B
Catalog No.:BCN7084
CAS No.:14930-96-2
- pp60 c-src (521-533) (phosphorylated)
Catalog No.:BCC5851
CAS No.:149299-77-4
- 1-Dehydroxy-23-deoxojessic acid
Catalog No.:BCN1663
CAS No.:149252-87-9
- De-4'-O-methylyangambin
Catalog No.:BCN1662
CAS No.:149250-48-6
- Neotripterifordin
Catalog No.:BCN7477
CAS No.:149249-32-1
- Arcaine sulfate
Catalog No.:BCC6631
CAS No.:14923-17-2
- Z-Ser(Tos)-OMe
Catalog No.:BCC2741
CAS No.:1492-52-0
- H-Abu-OH
Catalog No.:BCC3198
CAS No.:1492-24-6
- Benserazide HCl
Catalog No.:BCC4468
CAS No.:14919-77-8
- (±)-Myristoylcarnitine chloride
Catalog No.:BCC6698
CAS No.:14919-38-1
- UNC2881
Catalog No.:BCC5362
CAS No.:1493764-08-1
- Cidofovir dihydrate
Catalog No.:BCC4247
CAS No.:149394-66-1
- NE 100 hydrochloride
Catalog No.:BCC7573
CAS No.:149409-57-4
- Poncirin
Catalog No.:BCN2590
CAS No.:14941-08-3
- Traxillaside
Catalog No.:BCN6917
CAS No.:149415-62-3
- Hypocrellin C
Catalog No.:BCN3398
CAS No.:149457-83-0
- Ganoderic acid AM1
Catalog No.:BCN2441
CAS No.:149507-55-1
- Marmin
Catalog No.:BCN1665
CAS No.:14957-38-1
- Stachybotramide
Catalog No.:BCN6969
CAS No.:149598-71-0
- Impentamine dihydrobromide
Catalog No.:BCC7197
CAS No.:149629-70-9
- MOG (35-55)
Catalog No.:BCC3670
CAS No.:149635-73-4
- Vorinostat (SAHA, MK0683)
Catalog No.:BCC2145
CAS No.:149647-78-9
Vasodilatory and anti-inflammatory effects of the 1,2,3,4,6-penta-O-galloyl-beta-D-glucose (PGG) via a nitric oxide-cGMP pathway.[Pubmed:16253226]
Eur J Pharmacol. 2005 Nov 7;524(1-3):111-9.
Vasorelaxant and anti-inflammatory effects of a 1,2,3,4,6-penta-O-galloyl-beta-d-glucose (PGG) isolated from the root barks of Paeonia suffruticosa and possible mechanisms responsible were investigated. PGG induced a concentration-dependent relaxation of the phenylephrine-precontracted rat aorta. This effect disappeared with the removal of functional endothelium. Pretreatment of the aortic tissues with either N(G)-nitro-L-arginine methyl ester (L-NAME) or 1H-[1,2,4]-oxadiazole-[4,3-alpha]-quinoxalin-1-one (ODQ) inhibited the relaxation induced by PGG. Incubation of human umbilical vein endothelial cells (HUVECs) or carotid arteries isolated from rats with PGG increased the production of cGMP in a dose-dependent manner, but this effect was blocked by pretreatment with L-NAME and ODQ, respectively. PGG treatment attenuated tumor necrosis factor-alpha (TNF-alpha)-induced nuclear factor-kappaB (NF-kappaB) p65 translocation in human umbilical vein endothelial cells. In addition, PGG suppressed the expression levels of adhesion molecules including intracellular cell adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) induced by TNF-alpha. TNF-alpha-induced monocyte chemoattractant protein-1 (MCP-1) expression was also attenuated by addition of PGG. PGG treatment inhibited cellular adhesion of U937 cells onto human umbilical vein endothelial cells induced by TNF-alpha. Taken together, the present study suggests that PGG dilates vascular smooth muscle and suppresses the vascular inflammatory process via endothelium-dependent nitric oxide (NO)/cGMP signaling.
Inhibition of inducible nitric oxide synthase and cyclooxygenase-2 activity by 1,2,3,4,6-penta-O-galloyl-beta-D-glucose in murine macrophage cells.[Pubmed:14609132]
Arch Pharm Res. 2003 Oct;26(10):832-9.
Activated macrophages express inducible isoforms of nitric oxide synthase (iNOS) and cyclooxygenase (COX-2), and produce excessive amounts of nitric oxide (NO) and prostaglandin E2 (PGE2), which play key roles in the processes of inflammation and carcinogenesis. The root of Paeonia lactiflora Pall., and the root cortex of Paeonia suffruticosa Andr., are important Chinese crude drugs used in many traditional prescriptions. 1,2,3,4,6-penta-O-galloyl-beta-D-glucose (PGG) is a major bioactive constituent of both crude drugs. PGG has been shown to possess potent anti-oxidant, anti-mutagenic, anti-proliferative and anti-invasive effects. In this study, we examined the inhibitory effects of 1,2,3,4,6-penta-O-galloyl-beta-D-glucose (PGG) isolated from the root of Paeonia lactiflora Pall. on the COX-2 and iNOS activity in LPS-activated Raw 264.7 cells, COX-1 in HEL cells. To investigate the structure-activity relationships of gallate and gallic acid for the inhibition of iNOS and COX-2 activity, we also examined (-)-epigallocatechin gallate (EGCG), gallic acid, and gallacetophenone. The results of the present study indicated that PGG, EGCG, and gallacetophenone treatment except gallic acid significantly inhibited LPS-induced NO production in LPS-activated macrophages. All of the four compounds significantly inhibited COX-2 activity in LPS-activated macrophages. Among the four compounds examined, PGG revealed the most potent in both iNOS (IC50 approximately 18 microg/mL) and COX-2 inhibitory activity (PGE2: IC50 approximately 8 microg/mL and PGD2: IC50 approximately 12 microg/mL), respectively. Although further studies are needed to elucidate the molecular mechanisms and structure-activity relationship by which PGG exerts its inhibitory actions, our results suggest that PGG might be a candidate for developing anti-inflammatory and cancer chemopreventive agents.
Natural anti-diabetic compound 1,2,3,4,6-penta-O-galloyl-D-glucopyranose binds to insulin receptor and activates insulin-mediated glucose transport signaling pathway.[Pubmed:16137651]
Biochem Biophys Res Commun. 2005 Oct 21;336(2):430-7.
Insulin mimetics from natural sources are potential therapeutics that can act alone or supplement insulin and other anti-diabetic drugs in the prevention and treatment of diabetes. We recently reported the insulin-like glucose transport stimulatory activity of tannic acid (TA) in 3T3-L1 adipocytes. In this study, we find that chemically synthesized 1,2,3,4,6-penta-O-galloyl-beta-D-glucopyranose (beta-PGG), one of the components of TA, as well as its natural anomer alpha-PGG possess activity. Mechanistic studies in adipocytes with alpha-PGG, the more potent of the two anomers, reveal that inhibitors that block the insulin-mediated glucose transport, including one that inhibits the insulin receptor (IR), also completely abolish the glucose transport activated by alpha-PGG. In addition, alpha-PGG induces phosphorylation of the IR and Akt, activates PI 3-kinase, and stimulates membrane translocation of GLUT 4. Receptor binding studies indicate that alpha-PGG binds to the IR and affects the binding between insulin and IR by reducing the maximum binding of insulin to IR without significantly altering the binding affinity of insulin to IR. Western blotting analysis of the products of a cross-linking reaction suggests that alpha-PGG may bind to IR at a site located on the alpha-subunit of the receptor. Animal studies demonstrate that PGG reduces blood glucose levels and improves glucose tolerance in diabetic and obese animals. Our results suggest that PGG may serve as a model for the development of new types of anti-diabetic and anti-metabolic syndrome therapeutics.
In vitro antiviral activity of 1,2,3,4,6-penta-O-galloyl-beta-D-glucose against hepatitis B virus.[Pubmed:17015965]
Biol Pharm Bull. 2006 Oct;29(10):2131-4.
This study examined the antiviral activity of the root of Paeonia lactiflora PALL. Among the solvent fractions of the crude drug, the ethyl acetate fraction showed anti-hepatitis B virus (HBV) activity (IC50, 8.1 microg/ml) in an HBV-producing HepG2.2.15 cell culture system. The active anti-HBV principle was isolated and identified as 1,2,3,4,6-penta-O-galloyl-beta-D-glucose (PGG) from the crude drug by activity-guided fractionation. PGG isolated from P. lactiflora was examined for the inhibition of HBV multiplication by measurement of HBV DNA and hepatitis B surface antigen (HBsAg) levels in the extracellular medium of HepG2.2.15 cells after 8-d treatment. PGG decreased the level of extracellular HBV (IC50, 1.0 microg/ml) in a dose-dependent manner. PGG also reduced the HBsAg level by 25% at a concentration of 4 microg/ml. The gallate structure of PGG may play a critical role in the inhibition of anti-HBV activity. These results suggest that PGG could be a candidate for developing an anti-HBV agent.
Study of antimutagenic and antioxidant activities of gallic acid and 1,2,3,4,6-pentagalloylglucose from Pistacia lentiscus. Confirmation by microarray expression profiling.[Pubmed:17129579]
Chem Biol Interact. 2007 Jan 5;165(1):1-13.
In vitro antioxidant and antimutagenic activities of two polyphenols isolated from the fruits of Pistacia lentiscus was assessed. Antioxidant activity was determined by the ability of each compound to scavenge the free radical 1,1-diphenyl-2-picrylhydrazyl (DPPH*), to inhibit xanthine oxidase and to inhibit the lipid peroxidation induced by H(2)O(2) in K562 cell line. Antimutagenic activity was assayed with SOS chromotest using Escherichia coli PQ37 as tester strain and Comet assay using K562 cell line. 1,2,3,4,6-Pentagalloylglucose was found to be more effective to scavenge DPPH* radical and protect against lipid peroxidation. Moreover, these two compounds induced an inhibitory activity against nifuroxazide and aflatoxin B1 mutagenicity. The protective effect exhibited by these molecules was also determined by analysis of gene expression as response to an oxidative stress. For this purpose, we used a cDNA-microarray containing 82 genes related to cell defense, essentially represented by antioxidant and DNA repair proteins. We found that 1,2,3,4,6-pentagalloylglucose induced a decrease in the expression of 11 transcripts related to antioxidant enzymes family (GPX1, TXN, AOE372, SHC1 and SEPW1) and DNA repair (POLD1, APEX, POLD2, MPG, PARP and XRCC5). The use of Gallic acid, induced expression of TXN, TXNRD1, AOE372, GSS (antioxidant enzymes) and LIG4, POLD2, MPG, GADD45A, PCNA, RPA2, DDIT3, HMOX2, XPA, TDG, ERCC1 and GTF2H1 (DNA repair) as well as the repression of GPX1, SEPW1, POLD1 and SHC1 gene expression.


