(S)-(-)-5-IodowillardiineCAS# 140187-25-3 |
- I-BET-762
Catalog No.:BCC4474
CAS No.:1260907-17-2
- Bromodomain Inhibitor, (+)-JQ1
Catalog No.:BCC1132
CAS No.:1268524-70-4
- I-BET151 (GSK1210151A)
Catalog No.:BCC4476
CAS No.:1300031-49-5
- GSK1324726A
Catalog No.:BCC4038
CAS No.:1300031-52-0
- PFI-1 (PF-6405761)
Catalog No.:BCC2225
CAS No.:1403764-72-6
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 140187-25-3 | SDF | Download SDF |
| PubChem ID | 447196 | Appearance | Powder |
| Formula | C7H8IN3O4 | M.Wt | 325.06 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 25 mM in 1.1eq. NaOH | ||
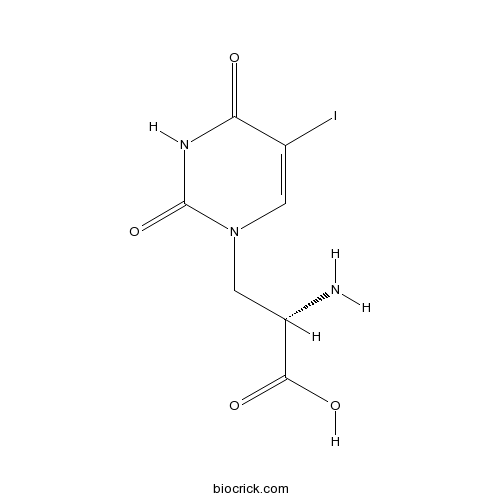
| Chemical Name | (2S)-2-amino-3-(5-iodo-2,4-dioxopyrimidin-1-yl)propanoic acid | ||
| SMILES | C1=C(C(=O)NC(=O)N1CC(C(=O)O)N)I | ||
| Standard InChIKey | AXXYLTBQIQBTES-BYPYZUCNSA-N | ||
| Standard InChI | InChI=1S/C7H8IN3O4/c8-3-1-11(2-4(9)6(13)14)7(15)10-5(3)12/h1,4H,2,9H2,(H,13,14)(H,10,12,15)/t4-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Demonstrates high affinity for the kainate receptor subtype hGluK1 (formerly hGluR5) (Ki = 0.24 nM) and 600-4000-fold selectivity over both the AMPA receptor subtypes and the homomeric kainate receptor hGluK2 (formerly hGluR6). |

(S)-(-)-5-Iodowillardiine Dilution Calculator

(S)-(-)-5-Iodowillardiine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0764 mL | 15.3818 mL | 30.7636 mL | 61.5271 mL | 76.9089 mL |
| 5 mM | 0.6153 mL | 3.0764 mL | 6.1527 mL | 12.3054 mL | 15.3818 mL |
| 10 mM | 0.3076 mL | 1.5382 mL | 3.0764 mL | 6.1527 mL | 7.6909 mL |
| 50 mM | 0.0615 mL | 0.3076 mL | 0.6153 mL | 1.2305 mL | 1.5382 mL |
| 100 mM | 0.0308 mL | 0.1538 mL | 0.3076 mL | 0.6153 mL | 0.7691 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- (S)-(-)-5-Fluorowillardiine
Catalog No.:BCC6596
CAS No.:140187-23-1
- Monnieriside A
Catalog No.:BCN7892
CAS No.:1401807-73-5
- Monnieriside G
Catalog No.:BCN7857
CAS No.:1401799-34-5
- (-)-Praeruptorin A
Catalog No.:BCN7664
CAS No.:14017-71-1
- Negundonorin A
Catalog No.:BCN7150
CAS No.:1401618-51-6
- ATPA
Catalog No.:BCC6940
CAS No.:140158-50-5
- Epimedin A1
Catalog No.:BCN5935
CAS No.:140147-77-9
- Pterisolic acid F
Catalog No.:BCN4840
CAS No.:1401419-90-6
- Pterisolic acid E
Catalog No.:BCN4841
CAS No.:1401419-89-3
- Pterisolic acid D
Catalog No.:BCN4839
CAS No.:1401419-88-2
- Pterisolic acid C
Catalog No.:BCN4838
CAS No.:1401419-87-1
- Pterisolic acid B
Catalog No.:BCN4843
CAS No.:1401419-86-0
- Mc-MMAD
Catalog No.:BCC1735
CAS No.:1401963-15-2
- Vc-MMAD
Catalog No.:BCC2032
CAS No.:1401963-17-4
- GSK2879552
Catalog No.:BCC6422
CAS No.:1401966-69-5
- Psiguadial D
Catalog No.:BCN7086
CAS No.:1402066-95-8
- Eupalinolide I
Catalog No.:BCN7367
CAS No.:1402067-84-8
- TUG-770
Catalog No.:BCC2018
CAS No.:1402601-82-4
- Boc-Lys(2-Cl-Z)-ol
Catalog No.:BCC2581
CAS No.:14028-05-8
- Amoxapine
Catalog No.:BCC4624
CAS No.:14028-44-5
- N-Methyllindcarpine
Catalog No.:BCN6218
CAS No.:14028-97-8
- NLG919
Catalog No.:BCC2325
CAS No.:1402836-58-1
- Chetomin
Catalog No.:BCC2432
CAS No.:1403-36-7
- Squalene-2,3-diol
Catalog No.:BCN6220
CAS No.:14031-37-9
Kainate receptors exhibit differential sensitivities to (S)-5-iodowillardiine.[Pubmed:9584222]
Mol Pharmacol. 1998 May;53(5):942-9.
Characterization of the role of kainate receptors in excitatory synaptic transmission has been hampered by a lack of subtype-selective pharmacological agents. (S)-5-Iodowillardiine (IW), an analog of willardiine [(S)-1-(2-amino-2-carboxyethyl)pyrimidine-2,4-dione], a heterocyclic amino acid found in Acacia and Mimosa seeds, was previously shown to be highly potent on native kainate receptors in dorsal root ganglion neurons. We examined the responses evoked by IW from recombinant homomeric and heteromeric kainate receptors expressed in human embryonic kidney 293 cells. IW potently elicited currents from glutamate receptor 5 (GluR5)-expressing cells, but showed no activity on homomeric GluR6 or GluR7 receptors. Co-expression of these receptor subunits with KA-2 subunits produced receptors that were weakly sensitive to IW. GluR5/KA-2 receptors had a higher EC50 value than homomeric GluR5 and exhibited a much faster recovery from desensitization. Finally, we found that the IW selectivity for GluR5 compared with GluR6 was determined by amino acid 721, which was previously shown to control alpha-amino-3-hydroxy-5-methyl-4-isoxazole-propionate sensitivity of these kainate receptor subunits. The pharmacological selectivity and commercial availability of IW suggests that this compound may be of use in characterizing the molecular constituents of native kainate receptor responses.
Synthesis of willardiine and 6-azawillardiine analogs: pharmacological characterization on cloned homomeric human AMPA and kainate receptor subtypes.[Pubmed:9357531]
J Med Chem. 1997 Oct 24;40(22):3645-50.
Both willardiine and azawillardiine analogs (18-28) have been reported to be potent and selective agonists for either AMPA or kainate receptors. We report here the novel synthesis and pharmacological characterization of a range of willardiine (18-23) and 6-azawillardiine (24-28) analogs on cells individually expressing human homomeric hGluR1, hGluR2, hGluR4, or hGluR5 receptors. Reaction of the sodium salts of substituted uracils (7-12) or 6-azauracils (13-16) with (S)-3-[(tert-butoxycarbonyl)amino]oxetan-2-one (17) in dry DMF, subsequent deprotection in TFA, and purification by ion-exchange chromatography gave mainly the willardiine analog in which alkylation took place on N1 of the uracil ring. We have investigated the subtype selectivity of these compounds by examining their binding affinity for homomeric hGluR1, -2, -4, or -5 (and hGluR6 in the case of 5-iodowillardiine (22)). From this study we have demonstrated that 22 has high affinity for hGluR5 and, compared to kainate, displays excellent selectivity for this receptor over both the AMPA receptor subtypes and the homomeric kainate receptor, hGluR6. 5-Fluorowillardiine (19) has higher affinity than AMPA for both homomeric hGluR1 and hGluR2 and compared to AMPA displays greater selectivity for AMPA receptor subtypes over the kainate receptor, hGluR5. Some structural features required for optimal activity at homomeric AMPA or kainate receptor subtypes have also been identified. It would appear that quite large lipophilic substituents at the 5-position of the uracil ring not only are accommodated by hGluR5 receptors but also lead to enhanced affinity for these receptors. In contrast to this, for optimal binding affinity to hGluR1, -2, or -4, smaller, electron-withdrawing substituents are required. For optimal activity at hGluR4 receptors a 6-aza-substituted willardiine is favored. The subtype-selective compounds described here are likely to be useful tools to probe the distribution and the physiological roles of the various glutamate receptor subunits in the central nervous system.
Willardiines differentiate agonist binding sites for kainate- versus AMPA-preferring glutamate receptors in DRG and hippocampal neurons.[Pubmed:7515954]
J Neurosci. 1994 Jun;14(6):3881-97.
Concentration jump responses to 5-substituted (S)-willardiines were recorded from dorsal root ganglion (DRG) and hippocampal neurons under voltage clamp. After block of desensitization by concanavalin-A, dose-response analysis for activation of kainate-preferring receptors in DRG neurons gave the potency sequence trifluoromethyl > iodo > bromo approximately chloro > nitro approximately cyano > kainate > methyl > fluoro > (R,S)-AMPA >> willardiine; EC50 values for the most and least potent willardiine derivatives, 5-trifluoromethyl (70 nM) and 5-fluoro (69 microM), differed 1000-fold. The potency sequence for equilibrium responses at AMPA-preferring receptors in hippocampal neurons was strikingly different from that obtained in DRG neurons: fluoro > cyano approximately trifluoromethyl approximately nitro > chloro approximately bromo > (R,S)-AMPA > iodo > willardiine > kainate > methyl. In hippocampal neurons EC50 values for the most and least potent willardiine derivatives, 5-fluoro (1.5 microM) and 5-methyl (251 microM), differed only 170-fold. Consistent with equilibrium potency measurements, in DRG neurons the kinetics of deactivation for willardiines, recorded following a return to agonist-free solution, were rapid for 5-fluoro (tau off = 43 msec) but slow for 5-iodo (tau off = 4.2 sec), while the opposite sequence was observed for hippocampal neurons, slow for 5-fluoro (tau off = 2.1 sec) and rapid for 5-iodo (tau off = 188 msec). The kinetics of recovery from desensitization showed comparable agonist- and cell-dependent differences. Structure-activity analysis for agonist responses recorded from DRG and hippocampal neurons suggests that for both kainate-preferring and AMPA-preferring receptors the binding of willardiines involves interactions with polar groups such that potency is related to ionization of the uracil ring, and hence the electron-withdrawing ability of the 5-position substituent. However, kainate-preferring receptors differ from AMPA-preferring receptors in possessing a lipophilic pocket that further enhances agonist potency by hydrophobic bonding of the 5-substituent. In contrast, AMPA-preferring receptors lack such a lipophilic site, and for 5-position substituents of the same electron-withdrawing ability, potency decreases with increase in size.
Activation and desensitization of AMPA/kainate receptors by novel derivatives of willardiine.[Pubmed:1371315]
J Neurosci. 1992 Feb;12(2):595-606.
Willardiine [(S)-1-(2-amino-2-carboxyethyl)pyrimidine-2,4-dione] is a naturally occurring heterocyclic excitatory amino acid present in the seeds of Acacia and Mimosa. A series of 5-substituted willardiines were synthesized in single enantiomeric forms and tested for activity at AMPA/kainate receptors, using whole-cell recording from mouse embryonic hippocampal neurons. The (S)- but not (R)-isomers of willardiine and 5-bromowillardiine were potent agonists, producing rapidly but incompletely desensitizing responses. At equilibrium, (S)-5-fluorowillardiine (EC50, 1.5 microM) was seven times more potent than (R,S)-AMPA (EC50, 11 microM) and 30 times more potent than willardiine (EC50, 45 microM); the potency sequence was fluoro greater than nitro greater than chloro approximately bromo greater than iodo greater than willardiine. Willardiines produce strikingly different degrees of desensitization: at saturating doses the equilibrium response to the weakly desensitizing agonist (S)-5-iodowillardiine was similar in amplitude to the response to kainate and 10 times larger than the response to the strongly desensitizing agonist (S)-willardiine. The desensitization sequence was fluoro greater than willardiine greater than nitro approximately chloro greater than bromo greater than iodo greater than kainate. Cross-desensitization experiments confirm that willardiines bind to the same receptors activated by kainate and AMPA, and show that both the rapidly desensitizing and equilibrium responses to willardiines are mediated by the same receptor: (S)-5-iodowillardiine blocked activation of the rapidly desensitizing response evoked by (S)-willardiine and (S)-5-fluorowillardiine, while the latter agonists blocked the equilibrium response to (S)-5-iodowillardiine. A slowly decaying inward tail current was recorded after a brief application of (S)-5-fluorowillardiine but not (S)-willardiine, consistent with a model in which willardiines bind with different affinity to desensitized receptors, such that following removal of agonist, receptors trapped in the desensitized state can return to the open state before dissociation of agonist terminates receptor activation. Willardiines are the first compounds characterized in which simple changes in molecular structure are associated with marked differences in the ability of agonists to produce desensitization of AMPA/kainate receptors.


