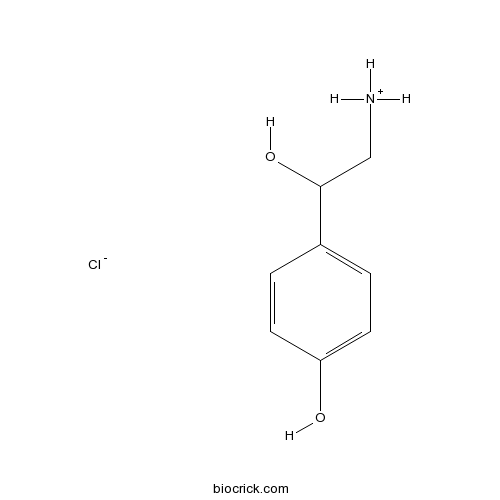
(+,-)-Octopamine HClTrace amine 1 (TA1) receptor agonist; invertebrate biogenic amine CAS# 770-05-8 |
- GW4064
Catalog No.:BCC4500
CAS No.:278779-30-9
- Chenodeoxycholic acid
Catalog No.:BCN2620
CAS No.:474-25-9
- XL335
Catalog No.:BCC4501
CAS No.:629664-81-9
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 770-05-8 | SDF | Download SDF |
| PubChem ID | 20592 | Appearance | Powder |
| Formula | C8H12ClNO2 | M.Wt | 189.64 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | (±)-<em>p</em>-Octopamine, Epirenor, Norfen | ||
| Solubility | DMSO : ≥ 100 mg/mL (527.31 mM) H2O : ≥ 50 mg/mL (263.66 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | 4-(2-amino-1-hydroxyethyl)phenol;hydrochloride | ||
| SMILES | C1=CC(=CC=C1C(CN)O)O.Cl | ||
| Standard InChIKey | PUMZXCBVHLCWQG-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C8H11NO2.ClH/c9-5-8(11)6-1-3-7(10)4-2-6;/h1-4,8,10-11H,5,9H2;1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Invertebrate biogenic amine neurotransmitter, related to noradrenalin, that is an adrenoceptor agonist. Stimulates lipolysis in mammalian adipocytes via activation of β3 receptors. Has dual effect on glucose transport in adipocytes: inhibits transport via β3 receptor activation but stimulates transport when oxidized by MAO. Also activates human α2A receptors, inhibiting subsequent cAMP production. |

(+,-)-Octopamine HCl Dilution Calculator

(+,-)-Octopamine HCl Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.2731 mL | 26.3657 mL | 52.7315 mL | 105.463 mL | 131.8287 mL |
| 5 mM | 1.0546 mL | 5.2731 mL | 10.5463 mL | 21.0926 mL | 26.3657 mL |
| 10 mM | 0.5273 mL | 2.6366 mL | 5.2731 mL | 10.5463 mL | 13.1829 mL |
| 50 mM | 0.1055 mL | 0.5273 mL | 1.0546 mL | 2.1093 mL | 2.6366 mL |
| 100 mM | 0.0527 mL | 0.2637 mL | 0.5273 mL | 1.0546 mL | 1.3183 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Octopamine (OA), a biogenic monoamine structurally related to noradrenaline, acts as a neurohormone, a neuromodulator and a neurotransmitter in invertebrates.
- D-(-)-Quinic acid
Catalog No.:BCN1029
CAS No.:77-95-2
- Triethyl citrate
Catalog No.:BCC9186
CAS No.:77-93-0
- Citric acid
Catalog No.:BCN5374
CAS No.:77-92-9
- Trometamol
Catalog No.:BCC4743
CAS No.:77-86-1
- Tigogenin
Catalog No.:BCN5327
CAS No.:77-60-1
- Tomatidine
Catalog No.:BCN2773
CAS No.:77-59-8
- Cedrol
Catalog No.:BCN8340
CAS No.:77-53-2
- Ursolic acid
Catalog No.:BCN4327
CAS No.:77-52-1
- Chlorthalidone
Catalog No.:BCC4649
CAS No.:77-36-1
- Gibberellins
Catalog No.:BCN2189
CAS No.:77-06-5
- Garcinone C
Catalog No.:BCN4322
CAS No.:76996-27-5
- Euphroside
Catalog No.:BCN6633
CAS No.:76994-07-5
- Dehydropachymic acid
Catalog No.:BCN3648
CAS No.:77012-31-8
- Hypocrellin A
Catalog No.:BCN3396
CAS No.:77029-83-5
- Knightinol
Catalog No.:BCN1913
CAS No.:77053-06-6
- Acetylknightinol
Catalog No.:BCN1914
CAS No.:77053-07-7
- Gadobutrol
Catalog No.:BCC4164
CAS No.:770691-21-9
- 6-O-trans-Feruloylcatalpol
Catalog No.:BCN4323
CAS No.:125205-48-3
- 2H-1-Benzopyran-5-ol
Catalog No.:BCN3581
CAS No.:770729-34-5
- MK-801 (Dizocilpine)
Catalog No.:BCC4591
CAS No.:77086-21-6
- (+)-MK 801 Maleate
Catalog No.:BCC4014
CAS No.:77086-22-7
- 1H-Indole-3-carboxylic acid
Catalog No.:BCN4324
CAS No.:771-50-6
- Fmoc-Sar-OH
Catalog No.:BCC3338
CAS No.:77128-70-2
- Fmoc-Phe(4-OMe)-OH,Fmoc-Tyr(Me)-OH
Catalog No.:BCC2634
CAS No.:77128-72-4
[Release of norfenefrine from sustained-release formulations by an in vitro dissolution model. Simulation of "drug levels" by calculation using pharmacokinetical constants and comparison with in vivo course of action (author's transl)].[Pubmed:7194653]
Arzneimittelforschung. 1981;31(2):353-6.
Drug release and dissolution behaviour of 1-(3'-hydroxyphenyl)-2-amino-ethan-1-ol hydrochloride (norfenefrine-HCl) from a sustained-release norfenefrine preparation (Esbuphon) were tested by an in vitro dissolution model in comparison with other norfenefrine formulations. By using pharmacokinetical constants, relative "drug levels" were calculated and compared with the activity curves from animal experiments. Conformities in time course were shown. Variations are discussed. The application of the model is justified if determination of serum concentration of norfenefrine cannot be done.
Is Na(+) required for the binding of dopamine, amphetamine, tyramine, and octopamine to the human dopamine transporter?[Pubmed:11919655]
Naunyn Schmiedebergs Arch Pharmacol. 2002 Apr;365(4):303-11.
The role of Na(+) in the recognition of blockers by the dopamine transporter is accomodated by a model with a cation site that overlaps with the blocker binding domain, and a distal Na(+) site that interacts with this cation site and perhaps with the blocker binding domain itself. The present study addresses the application of this model to the recognition of substrates by the dopamine transporter, focusing on conditions that should reveal a stimulatory effect, if present, of Na(+) on substrate binding. Recognition was studied via the inhibition of binding of [(3)H]WIN 35,428 (2beta-carbomethoxy-3beta-(4-fluorophenyl) [(3)H]tropane), a cocaine analog, to the human dopamine transporter in human embryonic kidney 293 cells. Little or no changes in binding were noted for dopamine, d-amphetamine, p-tyramine, or dl-octopamine by increasing [Na(+)] from 2 mM to 20 mM with co-varying Br(-), both at pH 7.4 and 7.0. In 74-mM Tris-HBr or -HCl, only dopamine and d-amphetamine showed binding increases upon raising Na(+), leveling off with NO(3)(-) or SO(4)(2-) but not Br(-) as anion at approximately 60 mM Na(+), consonant with a partly stimulatory action of Br(-). An Na(+) free, low 5-mM Tris-HEPES buffer was used for studying Na(+) curves truly starting at 0 mM, and, with SO(4)(2-) as the anion, no stimulation of binding by Na(+) was observed. This suggested that the stimulations observed in high (74 mM) Tris(+) buffer by Na(+) were not a direct effect of Na(+) but rather a disinhibitory effect of Na(+) in removing Tris(+) inhibition that depended upon substrate. Tris(+) IC(50) values in Na(+) free buffer were not lower for dopamine and d-amphetamine than p-tyramine and dl-octopamine. No evidence was found for a stronger inhibitory effect of Na(+) for dopamine and dl-octopamine potentially offsetting Tris(+) disinhibition. All results together support the existence of a substrate domain overlapping with a cation site that also binds Tris(+); a distal Na(+) site interacts with this cation site and with the substrate domain by negative allosterism and is additionally impacted by Cl(-). Importantly, interactions between sites vary with the type of substrate, and, in membrane preparations, Na(+) is not required for, or stimulatory to, the binding of any of the four substrates studied unlike the binding of the cocaine analog WIN 35,428.
Liquid chromatographic determination of biogenic amines in fermented foods after derivatization with 3,5-dinitrobenzoyl chloride.[Pubmed:10905732]
J Chromatogr A. 2000 Jun 9;881(1-2):517-30.
The reagent 3,5-dinitrobenzoyl chloride (DNBZ-Cl) was tested for pre-column derivatization of biogenic amines (BAs). Samples were derivatized within 3 min in 1 M NaOH at ambient temperature by adding 2-propanole and 50 mM DNBZ-Cl in acetonitrile. The reaction was terminated by addition of 2 M HCl. For high-performance liquid chromatography an encapsulated stationary reversed-phase and gradient elution using a ternary gradient system were used. The DNBZ derivatives were quantified by their UV-absorption at 260 nm. The structures of the derivatives were elucidated using coupling of HPLC with electrospray ionization mass spectrometry. Detection limits of BAs were approximately 124-864 microg l(-1) (injected amounts 203-1410 pg) at a signal-to-noise ratio of 3:1. The coefficients of determination were 0.989-0.996, with the exceptions of cadaverine (0.976) and serotonin (0.965). The method was applied to the quantitative determination of agmatine, cadaverine, histamine, octopamine, 2-phenylethylamine, putrescine, serotonin, spermidine, spermine, tryptamine and tyramine, in fermented cabbage juices, soy sauces, Misos (soy pastes), fermented fish sauces, and anchovy paste.
Down regulation of striatal dopamine receptors in experimental hepatic encephalopathy.[Pubmed:6403789]
Life Sci. 1983 Mar 28;32(13):1417-25.
Dopamine receptors were studied in striatal synaptosomes prepared from rat brain with hepatic encephalopathy induced by galactosamine-HCl and documented by visual evoked potential recordings. In order to further characterize the model, plasma amino acid levels and striatal catecholamines and octopamine levels were assayed. In agreement with previous reports in animal and in man, plasma amino acids were increased both in mild and severe stage of this pathology. Striatal levels of norepinephrine and dopamine fell during the development of coma while octopamine rose. Dopamine binding studies showed a decrease in the affinity during the mild stage and a reduction of receptor numbers in the severe stage of encephalopathy. The overall results, in the light of previous reports on GABA receptor studies, seem to indicate the presence in the development of encephalopathy of an imbalance between the dopaminergic and the GABAergic system leading to a prevalence of GABAergic inhibitory system.
A dopamine- and octopamine-sensitive adenylate cyclase in the nervous system of Octopus vulgaris.[Pubmed:1782763]
Comp Biochem Physiol B. 1991;100(4):805-8.
1. Adenylate cyclase activity was assayed in the optic lobe of Octopus vulgaris. 2. Both octopamine and dopamine stimulate the octopus adenylate cyclase, apparently by competing with the same receptor site. 3. (+/-)-2-Amino-6,7-dihydroxy-1,2,3,4-tetrahydronaphthalene-HBr (6,7-ADTN) and a number of phenylethanolamine derivatives stimulate the octopus adenylate cyclase activity. 4. The dopamine D-1 antagonists R(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl- 2,3,4,5-tetrahydro-1H-3-benzazepine-HCl (SCH-23390) and (+/-)-7-bromo-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H- 3-benzazepine-HCl (SKF-83566) are unable to antagonize the effects of dopamine and octopamine, and similarly ineffective is the agonist (+/-)-1-phenyl-2,3,4,5-tetrahydro-1H-3- benzazepine-7,8-diol-HCl (SKF-38393). 5. No detectable binding of labelled SCH-23390 occurs on membrane preparations from octopus optic lobe.
Dual action of octopamine on glucose transport into adipocytes: inhibition via beta3-adrenoceptor activation and stimulation via oxidation by amine oxidases.[Pubmed:11561068]
J Pharmacol Exp Ther. 2001 Oct;299(1):96-104.
Octopamine, which is closely related to norepinephrine, acts as a neurotransmitter in invertebrates and is a trace amine with undefined properties in vertebrates. The octopaminergic receptors identified in insects are targets of various pesticides but are absent in vertebrates. We have established that octopamine stimulates fat cell lipolysis in mammals via activation of beta3-adrenoceptors (ARs), whereas this amine has been described elsewhere as an alpha2-AR agonist and as a substrate for monoamine oxidase (MAO) or semicarbazide-sensitive amine oxidase (SSAO). Because we have recently reported that amine oxidase substrates promote glucose transport in rat and human adipocytes, the in vitro octopamine effects on lipolysis and glucose uptake were reassessed by using adipocytes from beta3-AR-deficient mice. The lipolytic effect and the counter-regulation of insulin action on glucose transport provoked by 0.1 to 1 mM octopamine or by 1 microM beta3-AR agonists found in control animals disappeared in adipocytes from beta3-AR-deficient mice. This revealed an insulin-like effect of octopamine on glucose uptake, which was dependent on its oxidation by MAO or SSAO, as was the case for tyramine and benzylamine, devoid of beta3-adrenergic agonism. Similarly, octopamine promoted glucose transport in human adipocytes and exhibited a weaker lipolytic stimulation than in rodent adipocytes. These findings indicate that, besides its lipolytic activity, octopamine exerts, at millimolar dose, dual effect on glucose transport in adipocytes: counteracting insulin action via beta3-AR activation and stimulating basal transport via its oxidation by MAO or SSAO.
Octopamine in invertebrates.[Pubmed:10515667]
Prog Neurobiol. 1999 Dec;59(5):533-61.
Octopamine (OA), a biogenic monoamine structurally related to noradrenaline, acts as a neurohormone, a neuromodulator and a neurotransmitter in invertebrates. It is present in relatively high concentrations in neuronal as well as in non-neuronal tissues of most invertebrate species studied. It functions as a model for the study of modulation in general. OA modulates almost every physiological process in invertebrates studied so far. Among the targets are peripheral organs, sense organs, and processes within the central nervous system. The known actions of OA in the central nervous system include desensitization of sensory inputs, influence on learning and memory, or regulation of the 'mood' of the animal. Together with tyramine, OA it is the only neuroactive non-peptide transmitter whose physiological role is restricted to invertebrates. This focussed the interest on the corresponding OA receptors. They are believed to be good targets for highly specific insecticides as they are not found in vertebrates. All octopamine receptors belong to the family of G-protein coupled receptors. Four of them could be distinguished using pharmacological tools. They show different coupling to second messenger systems including activation and inhibition of adenylyl cyclase, activation of phospholipase C and coupling to a chloride channel. Recently, octopamine receptors from molluscs and insects have been cloned. Further studies of all aspects of octopaminergic neurotransmission should give deeper insights into modulation of peripheral and sense organs and within the central nervous system in general.
Selective inhibition of adenylyl cyclase by octopamine via a human cloned alpha 2A-adrenoceptor.[Pubmed:9313925]
Br J Pharmacol. 1997 Sep;122(2):191-8.
1. In this study we have compared the abilities of the enantiomers of the structural isomers of the phenolamines, octopamine and synephrine, and the catecholamines, noradrenaline and adrenaline, to couple selectively a human cloned alpha 2A-adrenoceptor, stably expressed in a Chinese hamster ovary (CHO) cell line, to G-protein linked second messenger pathways mediating an increase and a decrease in cyclic AMP production. 2. The catecholamines couple the alpha 2A-adrenoceptor to both an increase and a decrease in the rate of cyclic AMP production. In the absence of pertussis toxin pretreatment both catecholamines tested showed a dose-dependent decrease with a maximum at 100 nM. After pertussis toxin pretreatment they both produced a dose-dependent increase in cyclic AMP production with a maximum at 10 microM. 3. The phenolamines, octopamine and synephrine were only able to couple the alpha 2A-adrenoceptor to a dose-dependent decrease in cyclic AMP production at concentrations up to 1 mM, with the synephrine isomers being more potent than the corresponding octopamine isomers. The meta-isomers of both phenolamines were more potent than the corresponding para-isomers and the (-)-enantiomers were more potent than the (+)-enantiomers. Thus, (-)-meta-synephrine [(-)-phenylephrine] was the most effective isomer tested with an observable decrease occurring between 100 nM and 1 microM. 4. The effects of octopamine and the catecholamines on the decrease in cyclic AMP production were additive at submaximal concentrations, whilst octopamine reduced the stimulant effect of submaximal concentrations of noradrenaline on cyclic AMP production after pertussis toxin pretreatment. 5. The time courses of the inhibitory effects of both meta-octopamine and noradrenaline were parallel and peaked after a 1 min exposure to the agonist. In contrast, the stimulant effects of noradrenaline after pertussis toxin pretreatment were of a much slower time course with a maximum effect occurring after a 5 min incubation period. 6. Since octopamine and synephrine occur naturally in, and are co-released with catecholamines from, mammalian tissues, the results of the present study suggest that the human cloned alpha 2A-adrenoceptor can be coupled selectively by different endogenous agonists to G-protein pathways mediating the regulation of adenylyl cyclase activity.


