TigecyclineGlycylcycline antibiotic CAS# 220620-09-7 |
- MGCD-265
Catalog No.:BCC2479
CAS No.:875337-44-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 220620-09-7 | SDF | Download SDF |
| PubChem ID | 54686904 | Appearance | Powder |
| Formula | C29H39N5O8 | M.Wt | 585.65 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | GAR-936 | ||
| Solubility | DMSO : 150 mg/mL (256.13 mM; Need ultrasonic) H2O : 50 mg/mL (85.38 mM; Need ultrasonic) | ||
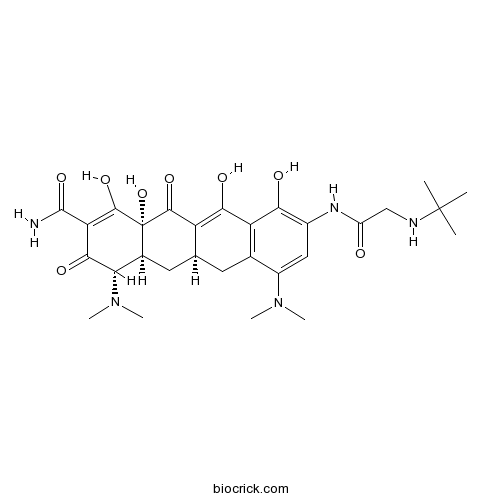
| Chemical Name | (4S,4aS,5aR,12aR)-9-[[2-(tert-butylamino)acetyl]amino]-4,7-bis(dimethylamino)-1,10,11,12a-tetrahydroxy-3,12-dioxo-4a,5,5a,6-tetrahydro-4H-tetracene-2-carboxamide | ||
| SMILES | CC(C)(C)NCC(=O)NC1=C(C2=C(CC3CC4C(C(=O)C(=C(C4(C(=O)C3=C2O)O)O)C(=O)N)N(C)C)C(=C1)N(C)C)O | ||
| Standard InChIKey | SOVUOXKZCCAWOJ-HJYUBDRYSA-N | ||
| Standard InChI | InChI=1S/C29H39N5O8/c1-28(2,3)31-11-17(35)32-15-10-16(33(4)5)13-8-12-9-14-21(34(6)7)24(38)20(27(30)41)26(40)29(14,42)25(39)18(12)23(37)19(13)22(15)36/h10,12,14,21,31,36-37,40,42H,8-9,11H2,1-7H3,(H2,30,41)(H,32,35)/t12-,14-,21-,29-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Tigecycline is a first-in-class, broad spectrum antibiotic with activity against antibiotic-resistant organisms.
Target: Antibacterial
Tigecycline is active against a broad range of gram-negative and gram-positive bacterial species including clinically important multidrug-resistant nosocomial and community-acquired bacterial pathogens. Tigecycline has been shown to inhibit the translation elongation step by binding to the ribosome 30S subunit and preventing aminoacylated tRNAs to accommodate in the ribosomal A site [1]. Tigecycline has also been found to be effective for the treatment of community- as well as hospital-acquired and ventilator-associated pneumonia and bacteremia, sepsis with shock and urinary tract infections. Tigecycline appears to be a valuable treatment option for the management of superbugs, especially where conventional therapy has failed [2].
Fifteen patients received tigecycline for 16 episodes of CPKP infection. The main infections were pneumonia (31%), urinary tract infection (31%), peritonitis (20%), catheter-related bacteraemia (12%), and meningitis (6%). Most infections were complicated with severe sepsis (44%), septic shock (12%), and/or bacteraemia (19%). The daily maintenance dose of tigecycline was 200 mg in 10 episodes and 100 mg in 6 episodes. The overall 30-day mortality rate was 25%. Univariate analysis showed that mortality was significantly associated (p < 0.01) with mean APACHE II and SOFA scores and the presence of immunosuppression, but not with the tigecycline dose [3]. References: | |||||

Tigecycline Dilution Calculator

Tigecycline Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.7075 mL | 8.5375 mL | 17.075 mL | 34.1501 mL | 42.6876 mL |
| 5 mM | 0.3415 mL | 1.7075 mL | 3.415 mL | 6.83 mL | 8.5375 mL |
| 10 mM | 0.1708 mL | 0.8538 mL | 1.7075 mL | 3.415 mL | 4.2688 mL |
| 50 mM | 0.0342 mL | 0.1708 mL | 0.3415 mL | 0.683 mL | 0.8538 mL |
| 100 mM | 0.0171 mL | 0.0854 mL | 0.1708 mL | 0.3415 mL | 0.4269 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Tigecycline is a glycylcycline antibiotic. It was developed in response to the growing prevalence of antibiotic resistance in bacteria such as Staphylococcus aureus and acinetobacter baumanii.
- GB 1a
Catalog No.:BCN7237
CAS No.:220611-41-6
- Diphyllin
Catalog No.:BCN8066
CAS No.:22055-22-7
- Caspase-3/7 Inhibitor I
Catalog No.:BCC1140
CAS No.:220509-74-0
- 1-Methyl-2-pentyl-4(1H)-quinolinone
Catalog No.:BCN4943
CAS No.:22048-98-2
- Leptomerine
Catalog No.:BCN1486
CAS No.:22048-97-1
- Kaempferol 5-methyl ether
Catalog No.:BCN3426
CAS No.:22044-80-0
- Polycephalin C
Catalog No.:BCN1852
CAS No.:220422-37-7
- ShK-Dap22
Catalog No.:BCC5990
CAS No.:220384-25-8
- 5-Epicanadensene
Catalog No.:BCN7349
CAS No.:220384-17-8
- DH 97
Catalog No.:BCC6973
CAS No.:220339-00-4
- TRO 19622
Catalog No.:BCC5288
CAS No.:22033-87-0
- 3,11,12-Trihydroxyspirovetiv-1(10)-en-2-one
Catalog No.:BCN1487
CAS No.:220328-04-1
- RJR-2403 oxalate
Catalog No.:BCC1902
CAS No.:220662-95-3
- Antisauvagine-30
Catalog No.:BCC5868
CAS No.:220673-95-0
- Oxonic acid potassium salt
Catalog No.:BCC4165
CAS No.:2207-75-2
- Ketoprofen
Catalog No.:BCC4430
CAS No.:22071-15-4
- Curlignan
Catalog No.:BCN3977
CAS No.:220736-54-9
- Aquifoliunine E-III
Catalog No.:BCN3096
CAS No.:220751-20-2
- Aminopurvalanol A
Catalog No.:BCC7249
CAS No.:220792-57-4
- NG,NG-Dimethylarginine dihydrochloride
Catalog No.:BCN1485
CAS No.:220805-22-1
- 7-Methoxy-8-Hydroxy-4-Methylcoumarin
Catalog No.:BCC8291
CAS No.:22084-94-2
- Thunalbene
Catalog No.:BCN3688
CAS No.:220862-05-5
- 2,3-O-Isopropylidenyl euscaphic acid
Catalog No.:BCN4944
CAS No.:220880-90-0
- GW5074
Catalog No.:BCC4391
CAS No.:220904-83-6
Molecular analysis of the ramRA locus in clinical Klebsiella pneumoniae isolates with reduced susceptibility to tigecycline.[Pubmed:28368073]
New Microbiol. 2017 Apr;40(2):135-138. Epub 2017 Apr 3.
Mutations in ramR, a negative regulator of ramA which stimulates transcription of acrA/-B encoding the multidrug efflux pump AcrAB-TolC, were recently shown to result in reduced susceptibility to Tigecycline in Klebsiella pneumoniae. We analysed six non-duplicate K. pneumoniae isolates with elevated MICs to Tigecycline. All isolates showed transcriptional up-regulation of ramA and acrB as demonstrated by Northern blot and quantitative real-time PCR. Sequencing of the ramR gene revealed deletions in five of the isolates and a premature stop codon in one isolate. Transformation of the wild-type ramR gene but not of any of the detected mutant ramR genes into a ramR-mutant K. pneumoniae strain restored Tigecycline susceptibility and repressed ramA and acrB transcription to wild type levels. Thus, our study confirms the role of inactivating mutations in the ramR gene in Tigecycline resistance.
Antimicrobial activity of tigecycline and cefoperazone/sulbactam tested against 18,386 Gram-negative organisms from Europe and the Asia-Pacific region (2013-2014).[Pubmed:28341098]
Diagn Microbiol Infect Dis. 2017 Jun;88(2):177-183.
A total of 18,386 organisms, including 13,224 Enterobacteriaceae, 3536 Pseudomonas aeruginosa, 1254 Acinetobacter spp., and 372Stenotrophomonas maltophilia were collected from Western Europe (WEU; n=10,021), Eastern Europe (EEU; n=4957), and the Asia-Pacific region (APAC; n=3408 [1052 from China]) in 2013-2014 as part of the SENTRY Antimicrobial Surveillance Program and tested by a reference broth microdilution method for susceptibility against Tigecycline, cefoperazone/sulbactam, and comparator agents. Overall, 95.3% of Enterobacteriaceae were susceptible (Tigecycline (MIC50/90, 0.12/1mug/mL) with regional EUCAST susceptibility rates of 94.8-97.8% (98.9-99.6% inhibited at Tigecycline (94.9% and 97.3% inhibited at Tigecycline. Cefoperazone/sulbactam inhibited 94.6/83.5/91.5% of Enterobacteriaceae at
Diet-Induced Obesity Does Not Alter Tigecycline Treatment Efficacy in Murine Lyme Disease.[Pubmed:28286500]
Front Microbiol. 2017 Feb 24;8:292.
Obese individuals more frequently suffer from infections, as a result of increased susceptibility to a number of bacterial pathogens. Furthermore, obesity can alter antibiotic treatment efficacy due to changes in drug pharmacokinetics which can result in under-dosing. However, studies on the treatment of bacterial infections in the context of obesity are scarce. To address this research gap, we assessed efficacy of antibiotic treatment in diet-induced obese mice infected with the Lyme disease pathogen, Borrelia burgdorferi. Diet-induced obese C3H/HeN mice and normal-weight controls were infected with B. burgdorferi, and treated during the acute phase of infection with two doses of Tigecycline, adjusted to the weights of diet-induced obese and normal-weight mice. Antibiotic treatment efficacy was assessed 1 month after the treatment by cultivating bacteria from tissues, measuring severity of Lyme carditis, and quantifying bacterial DNA clearance in ten tissues. In addition, B. burgdorferi-specific IgG production was monitored throughout the experiment. Tigecycline treatment was ineffective in reducing B. burgdorferi DNA copies in brain. However, diet-induced obesity did not affect antibiotic-dependent bacterial DNA clearance in any tissues, regardless of the Tigecycline dose used for treatment. Production of B. burgdorferi-specific IgGs was delayed and attenuated in mock-treated diet-induced obese mice compared to mock-treated normal-weight animals, but did not differ among experimental groups following antibiotic treatment. No carditis or cultivatable B. burgdorferi were detected in any antibiotic-treated group. In conclusion, obesity was associated with attenuated and delayed humoral immune responses to B. burgdorferi, but did not affect efficacy of antibiotic treatment.
Pharmacodynamics of tigecycline alone and in combination with colistin against clinical isolates of multidrug-resistant Acinetobacter baumannii in an in vitro pharmacodynamic model.[Pubmed:28315729]
Int J Antimicrob Agents. 2017 May;49(5):609-616.
The objectives of this study, which were based on the hypothesis of mutant prevention concentration (MPC), were to compare Tigecycline and colistin monotherapy and combination therapy against multidrug-resistant Acinetobacter baumannii (MDR-AB) and to identify changes in the susceptibility of the organism using an in vitro pharmacodynamic model. Human free-drug concentration profiles of colistin and Tigecycline used alone and in combination were simulated against four clinical MDR-AB isolates over 24 h. Pharmacodynamic activity was measured as log10 CFU/mL and as the area under the bactericidal curve (AUBC). The minimum inhibitory concentration (MIC) for all isolates was determined in triplicate by the broth microdilution method. All isolates grew to control levels in the Tigecycline and colistin monotherapy conditions, and the combination of colistin plus Tigecycline 100 mg every 12 h (q12h) or 50 mg q12h achieved a greater reduction in bacterial density than colistin alone (-2.65 +/- 1.73 or -2.09 +/- 1.47 vs. 0.98 +/- 0.64 log10 CFU/mL; P <0.01). Likewise, both combinations significantly reduced the AUBC compared with that achieved using colistin alone (106.9 +/- 24.5 or 117.7 +/- 23.5 vs. 168.1 +/- 14.2 log10 CFUh/mL; P <0.05). When Tigecycline or colistin monotherapy concentrations were below MPC, Tigecycline MICs increased 4-32-fold and colistin MICs increased >16-fold. No loss in susceptibility to Tigecycline was found with combination therapy. A combination of Tigecycline (high dose) and colistin may be an effective therapy to synergistically prevent the emergence of resistance during treatment of MDR-AB (Tigecycline MIC < 2 mg/L) infections.


