RJR-2403 oxalateNicotinic receptor agonist CAS# 220662-95-3 |
- RJR-2403
Catalog No.:BCC1901
CAS No.:15585-43-0
- Varenicline
Catalog No.:BCC4155
CAS No.:249296-44-4
- EVP-6124 hydrochloride
Catalog No.:BCC1567
CAS No.:550999-74-1
- EVP-6124
Catalog No.:BCC1566
CAS No.:550999-75-2
- LX-1031
Catalog No.:BCC1712
CAS No.:945976-76-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 220662-95-3 | SDF | Download SDF |
| PubChem ID | 23298272 | Appearance | Powder |
| Formula | C12H16N2O4 | M.Wt | 252.27 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Metanicotine, TC-2403 | ||
| Solubility | Soluble to 100 mM in water | ||
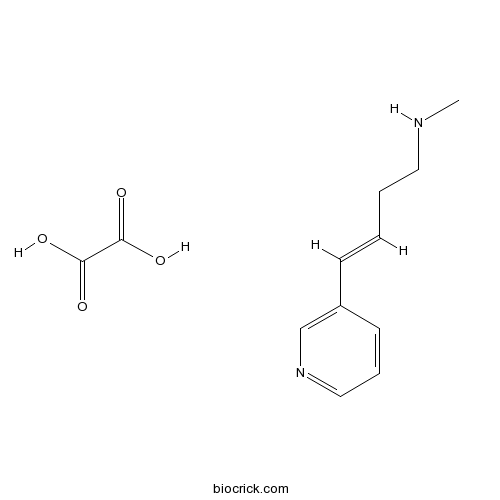
| Chemical Name | (E)-N-methyl-4-pyridin-3-ylbut-3-en-1-amine;oxalic acid | ||
| SMILES | CNCCC=CC1=CN=CC=C1.C(=O)(C(=O)O)O | ||
| Standard InChIKey | WTIZFOAIQXMQHC-DPZBITMOSA-N | ||
| Standard InChI | InChI=1S/C10H14N2.C2H2O4/c1-11-7-3-2-5-10-6-4-8-12-9-10;3-1(4)2(5)6/h2,4-6,8-9,11H,3,7H2,1H3;(H,3,4)(H,5,6)/b5-2+; | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | A neuronal nicotinic receptor agonist, showing high selectivity for the α4β2 subtype (Ki values are 26 and 36000 nM for α4β2 and α7 receptors respectively). Active in vivo. |

RJR-2403 oxalate Dilution Calculator

RJR-2403 oxalate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.964 mL | 19.82 mL | 39.6401 mL | 79.2801 mL | 99.1002 mL |
| 5 mM | 0.7928 mL | 3.964 mL | 7.928 mL | 15.856 mL | 19.82 mL |
| 10 mM | 0.3964 mL | 1.982 mL | 3.964 mL | 7.928 mL | 9.91 mL |
| 50 mM | 0.0793 mL | 0.3964 mL | 0.7928 mL | 1.5856 mL | 1.982 mL |
| 100 mM | 0.0396 mL | 0.1982 mL | 0.3964 mL | 0.7928 mL | 0.991 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
RJR-2403 oxalate((E)-Metanicotine oxalate; Rivanicline oxalate) is a neuronal nicotinic receptor agonist.RJR-2403 oxalate((E)-Metanicotine oxalate; Rivanicline oxalate) shows high selectivity for the α4β2 subtype (Ki values are 26 and 36000 nM for α4β2 and α7 receptors respectively). Active in vivo.
- Tigecycline
Catalog No.:BCC2499
CAS No.:220620-09-7
- GB 1a
Catalog No.:BCN7237
CAS No.:220611-41-6
- Diphyllin
Catalog No.:BCN8066
CAS No.:22055-22-7
- Caspase-3/7 Inhibitor I
Catalog No.:BCC1140
CAS No.:220509-74-0
- 1-Methyl-2-pentyl-4(1H)-quinolinone
Catalog No.:BCN4943
CAS No.:22048-98-2
- Leptomerine
Catalog No.:BCN1486
CAS No.:22048-97-1
- Kaempferol 5-methyl ether
Catalog No.:BCN3426
CAS No.:22044-80-0
- Polycephalin C
Catalog No.:BCN1852
CAS No.:220422-37-7
- ShK-Dap22
Catalog No.:BCC5990
CAS No.:220384-25-8
- 5-Epicanadensene
Catalog No.:BCN7349
CAS No.:220384-17-8
- DH 97
Catalog No.:BCC6973
CAS No.:220339-00-4
- TRO 19622
Catalog No.:BCC5288
CAS No.:22033-87-0
- Antisauvagine-30
Catalog No.:BCC5868
CAS No.:220673-95-0
- Oxonic acid potassium salt
Catalog No.:BCC4165
CAS No.:2207-75-2
- Ketoprofen
Catalog No.:BCC4430
CAS No.:22071-15-4
- Curlignan
Catalog No.:BCN3977
CAS No.:220736-54-9
- Aquifoliunine E-III
Catalog No.:BCN3096
CAS No.:220751-20-2
- Aminopurvalanol A
Catalog No.:BCC7249
CAS No.:220792-57-4
- NG,NG-Dimethylarginine dihydrochloride
Catalog No.:BCN1485
CAS No.:220805-22-1
- 7-Methoxy-8-Hydroxy-4-Methylcoumarin
Catalog No.:BCC8291
CAS No.:22084-94-2
- Thunalbene
Catalog No.:BCN3688
CAS No.:220862-05-5
- 2,3-O-Isopropylidenyl euscaphic acid
Catalog No.:BCN4944
CAS No.:220880-90-0
- GW5074
Catalog No.:BCC4391
CAS No.:220904-83-6
- 13-O-Cinnamoylbaccatin III
Catalog No.:BCN7344
CAS No.:220932-65-0
Gateways to clinical trials.[Pubmed:15834459]
Methods Find Exp Clin Pharmacol. 2005 Jan-Feb;27(1):49-77.
Gateways to Clinical Trials is a guide to the most recent clinical trials reported in current literature and congresses. The data in the following tables have been retrieved from the Clinical Trials Knowledge Area of Prous Science Integrity, the drug discovery and development portal, http://integrity.prous.com. This issue focuses on the following selection of drugs:[188Re]-HDD; A-179578, adalimumab, AK-602, albumin interferon alfa, alfimeprase, amelubant, anakinra, anti-CD2 MAb, APD-356, aripiprazole, atvogen; Bimatoprost, bimosiamose, BLP-25, brivaracetam; Caspofungin acetate, cilansetron, CMV vaccine (bivalent), conivaptan hydrochloride, Cypher; Darbepoetin alfa, darifenacin hydrobromide, D-D4FC, decitabine, dnaJP1, doranidazole, dronedarone hydrochloride; Efalizumab, efaproxiral sodium, emtricitabine, Endeavor, entecavir, erlotinib hydrochloride, escitalopram oxalate, etoricoxib, etravirine, ezetimibe; Fampridine, fenretinide, ferumoxtran-10, forodesine hydrochloride; Gantacurium chloride, gemi-floxacin mesilate, Glyminox, GW-501516; HBV-ISS, hepavir B, human insulin, HuMax-CD20, hyaluronic acid, HyCAMP; Icatibant, IDEA-070, IGN-311, imatinib mesylate, insulin detemir, insulin glargine, insulin glulisine; Lapatinib, lasofoxifene tartrate, LB-80380, liarozole fumarate, liposome encapsulated doxorubicin, lumiracoxib, LY-570310; MC-1, melatonin, merimepodib, metanicotine, midostaurin; Natalizumab, nicotine conjugate vaccine, NYVAC-HIV C; Patupilone, peginterferon alfa-2a, peginterferon alfa-2b, peginterferon alfa-2b/ribavirin, pelitinib, Peru-15, pexelizumab, PHP, pimecrolimus, prednisolone sodium metasulfobenzoate; Recombinant alfa1-antitrypsin (AAT), retigabine, rHA influenza vaccine, rifalazil, rofecoxib, rosiglitazone maleate/Metformin hydrochloride, rostaporfin, rosuvastatin calcium, rubitecan; Selenite sodium, semilente insulin, SMP-797, sorafenib; Talampanel, tenofovir disoproxil fumarate, TER-199, tiotropium bromide, torcetrapib, treprostinil sodium, TTA; ValboroPro, valdecoxib, val-mCyd, valtorcitabine dihydrochloride: XP-828L.
Antinociceptive and pharmacological effects of metanicotine, a selective nicotinic agonist.[Pubmed:10490929]
J Pharmacol Exp Ther. 1999 Oct;291(1):390-8.
Metanicotine [N-methyl-4-(3-pyridinyl)-3-butene-1-amine], a novel neuronal nicotinic agonist, was found to bind with high affinity (K(i) = 24 nM) to rat brain [(3)H]nicotine binding sites and it generalized to nicotine in a dose-dependent manner in the drug discrimination procedure. Metanicotine produced significant antinociceptive effects in mice and rats subjected to either acute thermal (tail-flick), mechanical (paw-pressure), chemical (para-phenylquinone), persistent (Formalin), and chronic (arthritis) pain stimuli. Metanicotine was about 5-fold less potent than nicotine in the tail-flick test after s.c administration, but slightly more potent after central administration. Its duration of action was longer than that of nicotine. Nicotinic antagonists, mecamylamine and dihydro-beta-erythroidine, blocked metanicotine-induced antinociception in the different pain models. However, the antinociceptive effect was not affected by pretreatment with either naloxone or by atropine, confirming that metanicotine exerts its antinociceptive effect via nicotinic rather than either opioid or muscarinic mechanisms. In contrast to nicotine, antinociceptive effects of metanicotine were observed at doses that had virtually no effect on spontaneous activity and body temperature in mice. These data indicate that metanicotine is a centrally acting neuronal nicotinic agonist with preferential antinociceptive effects in animals. Thus, metanicotine and related nicotinic agonists may have great potential for development as a new class of analgesics.
RJR-2403: a nicotinic agonist with CNS selectivity I. In vitro characterization.[Pubmed:8968366]
J Pharmacol Exp Ther. 1996 Dec;279(3):1413-21.
Increasing evidence for an involvement of nicotinic cholinergic systems in neurodegenerative disorders has stimulated the search for compounds with selectivity for CNS nicotinic ACh receptors (nAChRs). To this end, we have evaluated a number of nicotinic agonists for their ability to 1) bind to and up-regulate high-affinity nAChRs, 2) release [3H]-dopamine or induce 86Rb+ efflux in synaptosomes, 3) activate nAChRs in PC12 cells, 4) activate muscle-type nAChRs in human TE671/RD cells and 5) induce contraction of guinea pig ileum. Our results indicate that (E)-N-methyl-4-(3-pyridinyl)-3-butene-1-amine (RJR-2403) binds with high affinity to rat brain cortex (Ki = 26 +/- 3 nM). Functional studies show that RJR-2403 is comparable to nicotine in activating rat thalamic synaptosomes (EC50 = 732 +/- 155 nM and Emax = 91 +/- 8% for RJR-2403; EC50 = 591 +/- 120 nM and Emax = 100 +/- 25% for nicotine) but is one-tenth as potent in inducing dopamine release (EC50 = 938 +/- 172 nM and Emax = 82 +/- 5% for RJR-2403; EC50 = 100 +/- 25 nM and Emax = 100 +/- 13% for nicotine). At concentrations up to 1 mM, RJR-2403 does not significantly activate nAChRs in PC12 cells, muscle type nAChRs or muscarinic receptors. Dose-response curves for agonist-induced ileum contraction indicate that RJR-2403 is less than one-tenth as potent as nicotine with greatly reduced efficacy. RJR-2403 does not antagonize nicotine-stimulated muscle or ganglionic nAChR function (IC50 > 1 mM). Chronic exposure of M10 cells to RJR-2403 (10 microM) results in an up-regulation of high-affinity nAChRs phenomenologically similar to that seen with nicotine. These results suggest that RJR-2403 interacts with higher potency at CNS nAChR sub-types than at muscle, ganglionic or enteric nAChRs and has higher selectivity for CNS vs. muscle or ganglionic nAChRs than does nicotine.
RJR-2403: a nicotinic agonist with CNS selectivity II. In vivo characterization.[Pubmed:8968367]
J Pharmacol Exp Ther. 1996 Dec;279(3):1422-9.
We have evaluated the physiological and behavioral effects of the CNS-selective nicotinic agonist (E)-N-methyl-4-(3-pyridinyl) -3-butene-1-amine (RJR-2403) using a number of different methods, including 1) reversal of pharmacologically induced amnesia in a step-through passive avoidance paradigm, 2) radial arm maze performance in rats with chemically induced brain lesions, 3) changes in HR and blood pressure in rats and 4) changes in body temperature, Y-maze activity, acoustic startle response and respiration in mice. Our results indicate that RJR-2403 is equal to or better than nicotine on measures of CNS function and cognitive enhancement. Specifically, RJR-2403 significantly improved passive avoidance retention after scopolamine-induced amnesia and enhanced both working and reference memory in rats with ibotenic acid lesions of the forebrain cholinergic projection system in an 8-arm radial maze paradigm. By comparison, RJR-2403 was 15 to 30-fold less potent than nicotine in decreasing body temperature, respiration, Y-maze rears and crosses and acoustic startle response. RJR-2403 also demonstrated greatly reduced cardiovascular effects. RJR-2403 was approximately 10-fold less potent than nicotine in increasing HR and 20-fold less potent in increasing blood pressure. These results are consistent with in vitro data indicating this compound's high selectivity for CNS nicotinic ACh receptor subtypes relative to peripheral ganglionic and muscle-type nicotinic ACh receptors. Therefore, RJR-2403 may be a valuable tool for understanding the central and peripheral pharmacology of nicotinic cholinergic systems as well as a potential lead compound for the development of nicotinic therapeutics to treat neurological diseases where cholinergic neurotransmission has been compromised.


